
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 24.6, Problem 13P
Interpretation Introduction
a)
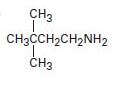
Interpretation:
To predict how to prepare the
Interpretation Introduction
b)
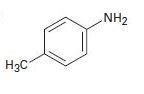
Interpretation:
To predict how to prepare the amines by using both Hofmann and Curtius rearrangements on a carboxylic acid derivative.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Complete the following esterification reaction by drawing the structural
formula of the product formed.
HOH
HO
i
catalyst
catalyst
OH
HO
(product has rum flavor)
(product has orange flavor)
The statements in the tables below are about two different chemical equilibria. The symbols have their usual meaning, for example AG stands for the standard
Gibbs free energy of reaction and K stands for the equilibrium constant.
In each table, there may be one statement that is faise because it contradicts the other three statements. If you find a false statement, check the box next to t
Otherwise, check the "no false statements" box under the table.
statement
false?
AG"1
no false statements:
statement
false?
AG-0
0
InK-0
0
K-1
0
AH-TAS
no false statements
2
Complete the following esterification reactions by drawing the line formulas of the carboxylic acid
and alcohol required to form the ester shown.
catalyst
catalyst
catalyst
apricot fragrance
Chapter 24 Solutions
Organic Chemistry
Ch. 24.1 - Name the following compounds:Ch. 24.1 - Prob. 2PCh. 24.1 - Prob. 3PCh. 24.3 - Prob. 4PCh. 24.3 - The benzylammonium ion (C6H5CH2NH3+) has pKa =...Ch. 24.4 - Prob. 6PCh. 24.5 - Prob. 7PCh. 24.6 - Prob. 8PCh. 24.6 - Prob. 9PCh. 24.6 - Show two methods for the synthesis of dopamine, a...
Ch. 24.6 - Prob. 11PCh. 24.6 - Prob. 12PCh. 24.6 - Prob. 13PCh. 24.7 - Prob. 14PCh. 24.7 - Prob. 15PCh. 24.8 - Prob. 16PCh. 24.8 - Propose syntheses of the following compounds from...Ch. 24.8 - How would you prepare the following compounds from...Ch. 24.8 - Prob. 19PCh. 24.9 - Draw an orbital picture of thiazole. Assume that...Ch. 24.9 - Prob. 21PCh. 24.9 - Prob. 22PCh. 24.9 - Which nitrogen atom in the hallucinogenic indole...Ch. 24.9 - Prob. 24PCh. 24.10 - Prob. 25PCh. 24.SE - Name the following amines, and identify each as...Ch. 24.SE - The following compound contains three nitrogen...Ch. 24.SE - Name the following amine, including R, S...Ch. 24.SE - Prob. 29VCCh. 24.SE - Predict the product(s) for each reaction below and...Ch. 24.SE - Predict the product(s) and provide the complete...Ch. 24.SE - Prob. 32MPCh. 24.SE - Predict the product(s) and provide the mechanism...Ch. 24.SE - The diazotization of aniline first involves the...Ch. 24.SE - Prob. 35MPCh. 24.SE - Prob. 36MPCh. 24.SE - Prob. 37MPCh. 24.SE - Prob. 38MPCh. 24.SE - Choline, a component of the phospholipids in cell...Ch. 24.SE - Prob. 40MPCh. 24.SE - -Amino acids can be prepared by the Strecker...Ch. 24.SE - Prob. 42MPCh. 24.SE - Prob. 43MPCh. 24.SE - Prob. 44MPCh. 24.SE - Propose a mechanism for the following reaction:Ch. 24.SE - Prob. 46MPCh. 24.SE - Name the following compounds:Ch. 24.SE - Prob. 48APCh. 24.SE - Prob. 49APCh. 24.SE - Prob. 50APCh. 24.SE - Histamine, whose release in the body triggers...Ch. 24.SE - Prob. 52APCh. 24.SE - Prob. 53APCh. 24.SE - Prob. 54APCh. 24.SE - Prob. 55APCh. 24.SE - Prob. 56APCh. 24.SE - Prob. 57APCh. 24.SE - Prob. 58APCh. 24.SE - Prob. 59APCh. 24.SE - Prob. 60APCh. 24.SE - Show the products from reaction of p-bromoaniline...Ch. 24.SE - Prob. 62APCh. 24.SE - How would you prepare the following compounds from...Ch. 24.SE - Prob. 64APCh. 24.SE - Phenacetin, a substance formerly used in...Ch. 24.SE - Prob. 66APCh. 24.SE - Draw the structure of the amine that produced the...Ch. 24.SE - Fill in the missing reagents a-e in the following...Ch. 24.SE - Prob. 69APCh. 24.SE - Prob. 70APCh. 24.SE - Deduce the structure of the compound with formula...Ch. 24.SE - Prob. 72APCh. 24.SE - Prob. 73APCh. 24.SE - Prob. 74APCh. 24.SE - Prob. 75APCh. 24.SE - Prob. 76APCh. 24.SE - Propose a structure for the product with formula...Ch. 24.SE - Prob. 78APCh. 24.SE - Prob. 79APCh. 24.SE - Prob. 80APCh. 24.SE - Prob. 81APCh. 24.SE - Prob. 82APCh. 24.SE - Prob. 83APCh. 24.SE - Prob. 84AP
Knowledge Booster
Similar questions
- Show the saponification products of the following ester: You don't need to draw in the Na+ cation. catalyst, A catalyst, A catalyst, Aarrow_forwardWhat would happen if the carboxylic acid and alcohol groups were on the same molecule? In essence, the molecule reacts with itself. Draw the structure of the products formed in this manner using the reactants below. If two functional groups interact with one another on the same molecule, this is called an “intramolecular" (within one) rather than "intermolecular" (between two or more) attack. OH OH catalyst OH HO catalyst catalyst HO OHarrow_forwardQ3: Write in the starting alkyl bromide used to form the following products. Include any reactants, reagents, and solvents over the reaction arrow. If more than one step is required, denote separate steps by using 1), 2), 3), etc. H OH racemic OH OH 5 racemicarrow_forward
- Draw the Lewis structure of the SO3-O(CH3)2 complex shown in the bottom right of slide 2in lecture 3-3 (“Me” means a CH3 group) – include all valence electron pairs and formal charges.From this structure, should the complex be a stable molecule? Explain.arrow_forwardPredict all organic product(s), including stereoisomers when applicable.arrow_forwardQ5: Propose a reasonable synthesis for the following decalin derivative. using only decalin and alkanes of 3 or fewer carbons. Decalin H3C HO க CH3arrow_forward
- 2Helparrow_forwardplease add appropriate arrows, and tell me clearly where to add arrows, or draw itarrow_forwardWhat I Have Learned Directions: Given the following reaction and the stress applied in each reaction, answer the question below. A. H2(g) + Cl2(g) 2 HCl(g) Stress applied: Decreasing the pressure 1. What is the Keq expression? 2. What will be the effect in the number of moles of HCl(g)? 3. What will be the Equilibrium Shift or the reaction? B. Fe3O4(s) + 4 H2(g) + heat 53 Fe(s) + 4 H₂O(g) Stress applied: Increasing the temperature 1. What is the Keq expression?. 2. What will be the effect in the volume of water vapor collected? 3. What will be the Equilibrium Shift or the reaction? C. 4 NH3(g) + 5 O2(g) 4 NO(g) + 6 H2O(g) + heat Stress applied: Increasing the volume of the container 1. What is the Keq expression?. 2. What will be the effect in the amount of H₂O? 3. What will be the Equilibrium Shift or the reaction?arrow_forward
- Consider the solubility products (Ksp values) for the following compounds:SrSO4 (Ksp = 7.6 x 10−7), BaSO4 (Ksp = 1.5 x 10−9), SrCO3 (Ksp = 7.0 x 10−10), BaCO3 (Ksp = 1.6 x 10−9)Which anion is the harder base, CO32− or SO42−? Justify your answer.arrow_forwardQ1: a) Arrange the compounds in order of decreasing pKa, highest first. ОН ΟΗ ῸΗ дон ОН ОН CI Brarrow_forward(4 pts - 2 pts each part) A route that can be taken to prepare a hydrophobic (water-repellent) aerogel is to start with trichloromethylsilane, CH3SiCl3 as the silicon source. a. What is the chemical reaction that this undergoes to form a product with Si-OH groups? Write as complete of a chemical equation as you can. CI CI-SI-CH3 CI b. The formation of a byproduct is what drives this reaction - what is the byproduct (if you didn't already answer it in part (a)) and how/why does it form?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning