
Chemistry & Chemical Reactivity
9th Edition
ISBN: 9781133949640
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 24.3, Problem 3Q
Interpretation Introduction
Interpretation:
The molecular geometry around the phosphorous atom in a phosphorothioate group has to be identified.
Concept introduction: In phosphorothioate group phosphrous atom bonded with surrounding atoms oxygen and sulphur as shown below.
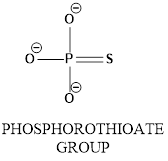
In the diagram shows
Tetrahedral geometry: A tetrahedral has a central atom which is surrounded by four other atoms. In tetrahedral geometry the bond angle is
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Homework 4
Chem 204
Dr. Hellwig
Consider this compound, which will be referred to as "your
compound".
a) Name your compound according to the IUPAC
system.
Include stereochemistry (E/Z/R/S)
H
CH3
CH3
What is the mechanism for this?
21.50 Determine the combinations of haloalkane(s) and alkoxide(s) that could be used to
synthesize the following ethers through Williamson ether synthesis.
(a)
(c)
(d)
(e)
(f)
H₂CO
Chapter 24 Solutions
Chemistry & Chemical Reactivity
Ch. 24.1 - Draw the Lewis structure for the tripeptide...Ch. 24.1 - Prob. 1RCCh. 24.1 - Prob. 2RCCh. 24.1 - Prob. 3RCCh. 24.2 - Prob. 1RCCh. 24.2 - Prob. 2RCCh. 24.2 - Prob. 3RCCh. 24.3 - What is the sequence of the strand of DNA...Ch. 24.3 - Prob. 2CYUCh. 24.3 - 1. Which breaks down more quickly in an aqueous...
Ch. 24.3 - Prob. 2RCCh. 24.3 - 3. Which amino acid is selected by the mRNA codon...Ch. 24.3 - Kynamro has the hydrogen bonding sequence:...Ch. 24.3 - The formula of Kynamro is...Ch. 24.3 - Prob. 3QCh. 24.4 - 1. Which of the following is not an example of a...Ch. 24.4 - Prob. 2RCCh. 24.5 - Prob. 1RCCh. 24.5 - Prob. 2RCCh. 24.5 - Prob. 1QCh. 24.5 - Prob. 2QCh. 24.5 - Prob. 3QCh. 24 - (a) Draw the Lewis structure for the amino acid...Ch. 24 - (a) Draw the Lewis structure for the amino acid...Ch. 24 - Prob. 3PSCh. 24 - Prob. 4PSCh. 24 - Draw Lewis structures for the two dipeptides that...Ch. 24 - Do the amino acid sequences: valine-asparagine and...Ch. 24 - Draw the Lewis structure for the tripeptide...Ch. 24 - Prob. 8PSCh. 24 - Prob. 9PSCh. 24 - Prob. 10PSCh. 24 - Prob. 11PSCh. 24 - Prob. 12PSCh. 24 - (a) Draw the structural formula for the sugar...Ch. 24 - (a) Draw the structural formula for the sugar -D-2...Ch. 24 - Prob. 15PSCh. 24 - Prob. 16PSCh. 24 - Given the following nucleotide sequence in DNA:...Ch. 24 - Given the following nucleotide sequence in DNA: 5'...Ch. 24 - Prob. 19PSCh. 24 - If a drop of oleic acid is added to a dish of...Ch. 24 - What structure do all steroids have in common?Ch. 24 - Prob. 22PSCh. 24 - Prob. 23PSCh. 24 - The chemical equation for the fermentation of...Ch. 24 - Prob. 25PSCh. 24 - Prob. 26PSCh. 24 - Prob. 27GQCh. 24 - Prob. 28GQCh. 24 - Prob. 29GQCh. 24 - Prob. 30GQCh. 24 - Prob. 31GQCh. 24 - There are 41 = 4 mononucleotides of DNA, there are...Ch. 24 - Prob. 33GQCh. 24 - The first step of the metabolic process known as...Ch. 24 - Prob. 35ILCh. 24 - Insulin is a protein important in the metabolism...Ch. 24 - Prob. 37SCQCh. 24 - Prob. 38SCQCh. 24 - Do the DNA sequences ATGC and CGTA represent the...Ch. 24 - Prob. 41SCQCh. 24 - Which of the following statements is/are true? (a)...
Knowledge Booster
Similar questions
- 1. Arrange the following in order of increasing bond energy (lowest bond energy first, highest bond energy last). Provide your rationale. C=C, C-F, C=C, C-N, C-C List the bond order for each example.arrow_forwardWhat is the major enolate formed when treated with LDA? And why that one?arrow_forward4. Calculate the total number of sigma bonds and total number of pi bonds in each of the following compounds. a. HH :D: +1 I H-N-C-C-O-H I H b. HH H Н :N=C-C-C=C-CEC-H :0: total o H-C-H H-C = `C-H I H. 11 H-C = C= CH H total o total π total π 1 Harrow_forward
- In the following reaction, what quantity in moles of CH₃OH are required to give off 4111 kJ of heat? 2 CH₃OH (l) + 3 O₂ (g) → 2 CO₂ (g) + 4 H₂O(g) ∆H° = -1280. kJarrow_forwardIndicate the processes in the dismutation of Cu2O.arrow_forward1. Consider these three reactions as the elementary steps in the mechanism for a chemical reaction. 2600 2400 2200 2000 1800 1600 1400 1200 1000 800 Potential Energy (kJ) 600 400 200 0 -200- -400 -600- -800 (i) Cl₂ (g) + Pt(s) → 2Cl (g) + Pt(s) (ii) Cl (g)+ CO (g) + Pt (s) → CICO (g) + Pt (s) Ea = 1550 kJ Ea = 2240 kJ (iii) Cl (g) + CICO (g) → Cl₂CO (g) Ea = 2350 kJ AH=-950 kJ ΔΗ = 575 ΚΙ AH=-825 kJ a. Draw the potential energy diagram for the reaction. Label the data points for clarity. The potential energy of the reactants is 600 kJ Reaction Progress b. What is the overall chemical equation? c. What is the overall change in enthalpy for the above chemical reaction? d. What is the overall amount of activation energy for the above chemical reaction? e. Which reaction intermediate would be considered a catalyst (if any) and why? f. If you were to add 2700kJ of energy to the reaction (e.g. 2700 kl of heat or electricity), would you be able to make the reaction reverse itself (i.e. have…arrow_forward
- draw the enolate anion and the carbonyl that would be needed to make this product through an aldol addition reaction.arrow_forwardDraw the Michael Adduct and the final product of the Robinson annulation reaction. Ignore inorganic byproducts.arrow_forwardDraw the Michael adduct and final product of the Robinson annulation reaction. Ignore inorganic byproductsarrow_forward
- Post Lab Questions. 1) Draw the mechanism of your Diels-Alder cycloaddition. 2) Only one isomer of product is formed in the Diels-Alder cycloaddition. Why? 3) Imagine that you used isoprene as diene - in that case you don't have to worry about assigning endo vs exo. Draw the "endo" and "exo" products of the Diels-Alder reaction between isoprene and maleic anhydride, and explain why the distinction is irrelevant here. 4) This does not hold for other dienes. Draw the exo and endo products of the reaction of cyclohexadiene with maleic anhydride. Make sure you label your answers properly as endo or exo. 100 °C Xylenes ??? 5) Calculate the process mass intensity for your specific reaction (make sure to use your actual amounts of reagent).arrow_forwardIndicate the product(s) A, B C and D that are formed in the reaction: H + NH-NH-CH [A+B] [C+D] hydrazonesarrow_forwardHow can you prepare a 6 mL solution of 6% H2O2, if we have a bottle of 30% H2O2?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

World of Chemistry, 3rd edition
Chemistry
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Brooks / Cole / Cengage Learning