
Concept explainers
(a)
Interpretation: To indicate whether the statement “pyruvate is the initial reactant for glycolysis” concerning glucose
Concept introduction: Pyruvate
A reactant is defined as the substance that is initially present in the
(b)
Interpretation: To indicate whether the statement “lactate is the initial reactant for gluconeogenesis” concerning glucose metabolic pathways is true or false.
Concept introduction: Lactate is the conjugate base of lactic acid. The structure of lactate is as follows:
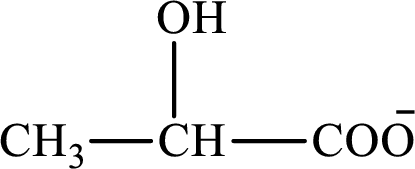
A reactant is defined as the substance that is initially present in the chemical reaction and gets consumed to form a new substance.
Gluconeogenesis is an eleven-step pathway in which glucose is produced from non-carbohydrate substances.
(c)
Interpretation: To indicate whether the statement “glycogen is the initial reactant for glycogenolysis” concerning glucose metabolic pathways is true or false.
Concept introduction: Glucose is a monosaccharide with the molecular formula
A reactant is defined as the substance that is initially present in the chemical reaction and gets consumed to form a new substance.
(d)
Interpretation: To indicate whether the statement “
Concept introduction: Glucose is a monosaccharide with the molecular formula
A reactant is defined as the substance that is initially present in the chemical reaction and gets consumed to form a new substance.
Want to see the full answer?
Check out a sample textbook solution
Chapter 24 Solutions
General, Organic, and Biological Chemistry
- Name the following molecules using iupacarrow_forwardWrite the amididation reaction mechanism of a-aminophenol and acetic acid to produce acetaminophenarrow_forwardFor the condensation reaction between Alamine and histamine, please help me write the amididation reaction mechanism. Then write the three letter code for the product of the reaction, then write the one letter code for the product of the reaction. arrow_forward
- Using the chart describe the change from cystine to tyrosine and its impact on the protein. Using the chart describe the change from histidine to aspartic acid and its impact on the protein.arrow_forwardHow to get the predicted product of this reaction belowarrow_forwardPlease help me fill out the chart then using the chart describe the change from cystine to tyrosine and its impact on the protein. Then using the chart describe the change from histidine to aspartic acid.arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





