
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 23.8, Problem 17P
Write out steps to show how each of the following pairs of compounds can be separated by an extraction procedure.
a. 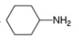 and
and 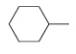
b. 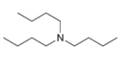 and
and 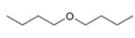
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1.57 Draw all reasonable resonance structures for the following cation. Then draw the
resonance hybrid.
For the two questions below, draw the mechanism and form the major product.
Indicate similarities and differences between natural, exchanged and pillared clays.
Chapter 23 Solutions
Organic Chemistry (6th Edition)
Ch. 23.2 - Prob. 1PCh. 23.3 - Prob. 2PCh. 23.3 - Prob. 3PCh. 23.4 - Prob. 4PCh. 23.6 - Prob. 7PCh. 23.6 - Prob. 8PCh. 23.6 - Prob. 9PCh. 23.6 - Prob. 10PCh. 23.6 - Prob. 11PCh. 23.6 - Prob. 12P
Ch. 23.6 - Prob. 13PCh. 23.6 - Prob. 14PCh. 23.6 - Prob. 15PCh. 23.8 - Prob. 16PCh. 23.8 - Problem 25.18
Write out steps to show how each of...Ch. 23.9 - Prob. 18PCh. 23.9 - Prob. 19PCh. 23.9 - Prob. 20PCh. 23.9 - Problem 25.22
Which nitrogen atom in each compound...Ch. 23.9 - Prob. 22PCh. 23.10 - Prob. 23PCh. 23.10 - Prob. 24PCh. 23.11 - Prob. 25PCh. 23.11 - Prob. 26PCh. 23.11 - Problem 25.28
Draw the major product formed in...Ch. 23.12 - Prob. 28PCh. 23.13 - Prob. 29PCh. 23 - Give a systematic or common name for each...Ch. 23 - Prob. 38PCh. 23 - 25.40 How many stereogenic centers are present in...Ch. 23 - 25.41 Rank the compounds in each group in order of...Ch. 23 - 25.42 Decide which atom in each molecule is most...Ch. 23 - Prob. 47PCh. 23 - Prob. 48PCh. 23 - Prob. 49PCh. 23 - 25.52 Draw the products formed when methylaniline ...Ch. 23 - Prob. 53PCh. 23 - 25.60 A chiral amine A having the configuration...Ch. 23 - Prob. 68PCh. 23 - Prob. 69PCh. 23 - Prob. 70PCh. 23 - 25.70 Devise a synthesis of each biologically...Ch. 23 - 25.71 Devise a synthesis of each compound from...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Show work. don't give Ai generated solutionarrow_forwardIn intercalation compounds, their sheets can be neutral or have a negative or positive charge, depending on the nature of the incorporated species and its structure. Is this statement correct?arrow_forwardThis thermodynamic cycle describes the formation of an ionic compound MX2 from a metal element M and nonmetal element X in their standard states. What is the lattice enthalpy of MX2 ? What is the enthalpy formation of MX2 ? Suppose both the heat of sublimation of M and the ionization enthalpy of M were smaller. Would MX2 be more stable? Or less? or impossible to tell without more information?arrow_forward
- I need to make 25mL of solution with the stocks shown below. How would I calculate the math?arrow_forwardWe are practicing calculating for making solutions. How would I calculate this?arrow_forwardBr. , H+ .OH Mg ether solvent H+, H₂O 17. Which one of the compounds below is the final product of the reaction sequence shown above? HO A HO HO OH D B OH HO OH C OH HO OH Earrow_forward
- 8:57 PM Sun Jan 26 Content ← Explanation Page X Content X ALEKS Jade Nicol - Le A https://www-av C www-awa.aleks.com O States of Matter Understanding consequences of important physical properties of liquids ? QUESTION Liquid A is known to have a lower viscosity and lower surface tension than Liquid B. Use these facts to predict the result of each experiment in the table below, if you can. experiment Liquid A and Liquid B are each pumped through tubes with an inside diameter of 27.0 mm, and the pressures PA and PB needed to produce a steady flow of 2.4 mL/s are measured. 25.0 mL of Liquid A are poured into a beaker, and 25.0 mL of Liquid B are poured into an identical beaker. Stirrers in each beaker are connected to motors, and the forces FA and FB needed to stir each liquid at a constant rate are measured. predicted outcome OPA will be greater than PB OPA will be less than PB OPA will be equal to PB It's impossible to predict whether PA or PB will be greater without more information.…arrow_forwardShow work. Don't give Ai generated solutionarrow_forward5. Please draw in the blanks the missing transition states and the correlated products. Explicitly display relevant absolute stereochemical configuration. MeOH I OMe H Endo transition state, dienophile approaching from the bottom of diene + H ཎྞཾ ཌཱརཱ༔,_o OMe H H OMe Endo transition state, dienophile approaching from the top of diene or from the bottom but horizontally flipped (draw one) + Exo transition state, dienophile approaching from the top of diene or from the bottom but horizontally flipped (draw one) Exo transition state, dienophile approaching from the top of diene or from the bottom but horizontally flipped (draw one) MeO H H MeO H MeO H MeO H Harrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning


Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole

Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY