
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 23.9, Problem 21P
Which nitrogen atom in each compound is more basic?
a. 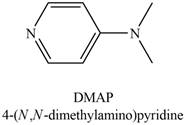 b.
b. 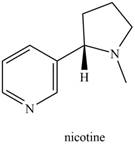
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Identify any polar covalent bonds in epichlorohydrin with S+ and 8- symbols in the appropriate locations. Choose the correct answer
below.
Η
H's+
6Η Η
Η
Η
Η
Ηδ
Η
Ο
Ο
HH
+Η Η
+Η Η
Η
-8+
CI
H
H:O::::H
H
H
HH
H::O:D:D:H
HH
HH
H:O:D:D:H
..
HH
H:O:D:D:H
H H
Select the correct Lewis dot structure for the following compound:
CH3CH2OH
Rank the following compounds in order of decreasing boiling point.
ннннн
-С-С-Н
.
н-с-
ННННН
H
ΗΤΗ
НННН
TTTĪ
н-с-с-с-с-о-н
НННН
НН
C' Н
н-с-с-с-с-н
НН
||
Ш
НННН
H-C-C-C-C-N-H
ННННН
IV
Chapter 23 Solutions
Organic Chemistry (6th Edition)
Ch. 23.2 - Prob. 1PCh. 23.3 - Prob. 2PCh. 23.3 - Prob. 3PCh. 23.4 - Prob. 4PCh. 23.6 - Prob. 7PCh. 23.6 - Prob. 8PCh. 23.6 - Prob. 9PCh. 23.6 - Prob. 10PCh. 23.6 - Prob. 11PCh. 23.6 - Prob. 12P
Ch. 23.6 - Prob. 13PCh. 23.6 - Prob. 14PCh. 23.6 - Prob. 15PCh. 23.8 - Prob. 16PCh. 23.8 - Problem 25.18
Write out steps to show how each of...Ch. 23.9 - Prob. 18PCh. 23.9 - Prob. 19PCh. 23.9 - Prob. 20PCh. 23.9 - Problem 25.22
Which nitrogen atom in each compound...Ch. 23.9 - Prob. 22PCh. 23.10 - Prob. 23PCh. 23.10 - Prob. 24PCh. 23.11 - Prob. 25PCh. 23.11 - Prob. 26PCh. 23.11 - Problem 25.28
Draw the major product formed in...Ch. 23.12 - Prob. 28PCh. 23.13 - Prob. 29PCh. 23 - Give a systematic or common name for each...Ch. 23 - Prob. 38PCh. 23 - 25.40 How many stereogenic centers are present in...Ch. 23 - 25.41 Rank the compounds in each group in order of...Ch. 23 - 25.42 Decide which atom in each molecule is most...Ch. 23 - Prob. 47PCh. 23 - Prob. 48PCh. 23 - Prob. 49PCh. 23 - 25.52 Draw the products formed when methylaniline ...Ch. 23 - Prob. 53PCh. 23 - 25.60 A chiral amine A having the configuration...Ch. 23 - Prob. 68PCh. 23 - Prob. 69PCh. 23 - Prob. 70PCh. 23 - 25.70 Devise a synthesis of each biologically...Ch. 23 - 25.71 Devise a synthesis of each compound from...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Rank the following compounds in order of decreasing dipole moment. |>||>||| ||>|||>| |>|||>|| |||>||>| O ||>>||| H F H F H c=c || H c=c F F IIIarrow_forwardchoose the description that best describes the geometry for the following charged species ch3-arrow_forwardWhy isn't the ketone in this compound converted to an acetal or hemiacetal by the alcohol and acid?arrow_forward
- What is the approximate bond angle around the nitrogen atom? HNH H Harrow_forwardOH 1. NaOCH2CH3 Q 2. CH3CH2Br (1 equiv) H3O+ Select to Draw 1. NaOCH2 CH3 2. CH3Br (1 equiv) heat Select to Edit Select to Drawarrow_forwardComplete and balance the following half-reaction in acidic solution. Be sure to include the proper phases for all species within the reaction. S₂O₃²⁻(aq) → S₄O₆²⁻(aq)arrow_forward
- Q Select to Edit NH3 (CH3)2CHCI (1 equiv) AICI 3 Select to Draw cat. H2SO4 SO3 (1 equiv) HO SOCl2 pyridine Select to Edit >arrow_forwardComplete and balance the following half-reaction in basic solution. Be sure to include the proper phases for all species within the reaction. Zn(s) → Zn(OH)₄²⁻(aq)arrow_forwardb. ὋΗ CH3CH2OH H2SO4arrow_forward
- For the reaction A (g) → 3 B (g), Kp = 0.379 at 298 K. What is the value of ∆G for this reaction at 298 K when the partial pressures of A and B are 5.70 atm and 0.250 atm?arrow_forward14. Calculate the concentrations of Ag+, Ag(S2O3), and Ag(S2O3)23- in a solution prepared by mixing 150.0 mL of 1.00×10-3 M AgNO3 with 200.0 mL of 5.00 M Na2S2O3 Ag+ + S20 Ag(S203)¯ K₁ = 7.4 × 108 Ag(S203)¯ + S20¯ = Ag(S203) K₂ = 3.9 x 104arrow_forwardΗΝ, cyclohexanone pH 4-5 Draw Enamine I I CH3CH2Br THF, reflux H3O+ I Drawing Draw Iminium Ionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY