
Organic Chemistry
5th Edition
ISBN: 9780078021558
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 23, Problem 23.5P
Which
a. 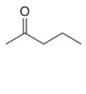 b.
b. 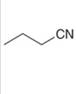 c.
c. 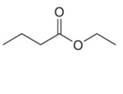 d.
d. 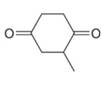
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Indicate what metal clusters are.
55. The photoelectric threshold energy for ytterbium
metal is 4.16 × 10-19 J/atom.
a. Calculate the wavelength of light that this
energy corresponds to (in nm).
b. Which region of the electromagnetic spectrum
does this wavelength fall in?
c. Would light of wavelength 490 nm produce a
photoelectric effect in ytterbium? Why or why
not?
14.50 Explain why methyl vinyl ether (CH2=CHOCH 3) is not a reactive
dienophile in the Diels-Alder reaction.
Chapter 23 Solutions
Organic Chemistry
Ch. 23 - Problem 23.1 Draw the enol or keto tautomer(s) of...Ch. 23 - Prob. 23.2PCh. 23 - Problem 23.3 When phenylacetaldehyde is dissolved...Ch. 23 - Prob. 23.4PCh. 23 - Problem 23.5 Which bonds in the following...Ch. 23 - Prob. 23.6PCh. 23 - Prob. 23.7PCh. 23 - Prob. 23.8PCh. 23 - Prob. 23.9PCh. 23 - Prob. 23.10P
Ch. 23 - Problem 23.11 Draw the products of each...Ch. 23 - Problem 23.12 Draw the products of each reaction....Ch. 23 - Prob. 23.13PCh. 23 - Prob. 23.14PCh. 23 - Prob. 23.15PCh. 23 - Prob. 23.16PCh. 23 - Prob. 23.17PCh. 23 - Problem 23.18 How can pentan-2-one be converted...Ch. 23 - Problem 23.19 Identify A, B, and C, intermediates...Ch. 23 - Problem 23.20 Which of the following compounds...Ch. 23 - Problem 23.21 Draw the products of each...Ch. 23 - Prob. 23.22PCh. 23 - Prob. 23.23PCh. 23 - Prob. 23.24PCh. 23 - Prob. 23.25PCh. 23 - Prob. 23.26PCh. 23 - Prob. 23.27PCh. 23 - Prob. 23.28PCh. 23 - 23.29 Draw enol tautomer(s) for each compound....Ch. 23 - 22.30 The cis ketone A is isomerized to a trans...Ch. 23 - 23.31 Draw enol tautomer(s) for each compound.
...Ch. 23 - Prob. 23.32PCh. 23 - Prob. 23.33PCh. 23 - Prob. 23.34PCh. 23 - 23.35 Rank the labeled protons in each compound in...Ch. 23 - Prob. 23.36PCh. 23 - Prob. 23.37PCh. 23 - 23.38 Acyclovir is an effective antiviral agent...Ch. 23 - 23.39 Explain why forms two different alkylation...Ch. 23 - Prob. 23.40PCh. 23 - 23.41 Acid-catalyzed bromination of pentanone ...Ch. 23 - 23.42 Draw a stepwise mechanism for the following...Ch. 23 - Prob. 23.43PCh. 23 - Prob. 23.44PCh. 23 - 23.45 Devise a synthesis of valproic acid , a...Ch. 23 - 23.46 Synthesize each compound from diethyl...Ch. 23 - Prob. 23.47PCh. 23 - Prob. 23.48PCh. 23 - Prob. 23.49PCh. 23 - 23.50 Draw the organic products formed in each...Ch. 23 - 23.51 Draw the products formed (including...Ch. 23 - Prob. 23.52PCh. 23 - Prob. 23.53PCh. 23 - 23.54 Clopidogrel is the generic name for Plavix,...Ch. 23 - 23.55 What reaction conditions—base, solvent, and...Ch. 23 - Prob. 23.56PCh. 23 - 23.57 Draw a stepwise mechanism showing how two...Ch. 23 - 23.58 Draw a stepwise mechanism for the following...Ch. 23 - Prob. 23.59PCh. 23 - 23.60 Draw stepwise mechanisms illustrating how...Ch. 23 - Prob. 23.61PCh. 23 - Prob. 23.62PCh. 23 - 23.63 Synthesize each compound from cyclohexanone...Ch. 23 - Prob. 23.64PCh. 23 - Prob. 23.65PCh. 23 - 23.66 Synthesize (Z)-hept-5-en-2-one from ethyl...Ch. 23 - Prob. 23.67PCh. 23 - 23.68 Capsaicin, the spicy component of hot...Ch. 23 - 23.69 Treatment of W with , followed by , affords...Ch. 23 - Prob. 23.70PCh. 23 - Prob. 23.71PCh. 23 - Prob. 23.72PCh. 23 - Prob. 23.73PCh. 23 - Prob. 23.74P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Show work with explanation needed. don't give Ai generated solutionarrow_forward14.49 From what you have learned about the reaction of conjugated dienes in Section 14.10, predict the products of each of the following electrophilic additions. a. H₂O H2SO4 Br2 b. H₂Oarrow_forward14.46 Draw a stepwise mechanism for the following reaction. HBr ROOR Br + Brarrow_forward
- Show work..don't give Ai generated solution....arrow_forward14.47 Addition of HCI to alkene X forms two alkyl halides Y and Z. exocyclic C=C X HCI CI Y + CI Z a. Label Y and Z as a 1,2-addition product or a 1,4-addition product. b.Label Y and Z as the kinetic or thermodynamic product and explain why. c. Explain why addition of HCI occurs at the indicated C=C (called an exocyclic double bond), rather than the other C=C (called an endocyclic double bond).arrow_forward14.44 Ignoring stereoisomers, draw all products that form by addition of HBr to (E)-hexa-1,3,5-triene.arrow_forward
- Include stereochemistry Leven though the solutions manual does 14.43 Draw the products formed when each compound is treated with one not) equivalent of HBr. a. b. C.arrow_forward14.41 Label each pair of compounds as stereoisomers, conformations, or constitutional isomers: (a) A and B; (b) A and C; (c) A and D; (d) C and D. A B C Darrow_forwardSteps and detailed explanation for work. Thanks!arrow_forward
- 14.39 Draw the structure of each compound. a. (Z)-penta-1,3-diene in the s-trans conformation b. (2E,4Z)-1-bromo-3-methylhexa-2,4-diene c. (2E,4E,6E)-octa-2,4,6-triene d. (2E,4E)-3-methylhexa-2,4-diene in the s-cis conformationarrow_forwardPLEASE ANSWER ALL PARTS!!arrow_forwardpls help on all, inlcude all steps.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY