
Concept explainers
Draw the organic products formed in each reaction.
a.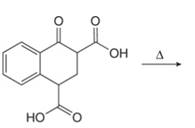 e.
e.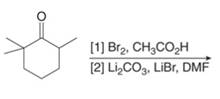
b.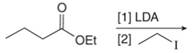 f.
f.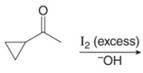
c.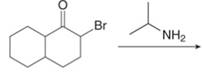 g.
g.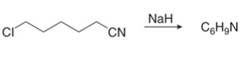
d.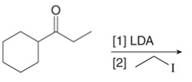 h.
h.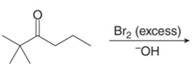
(a)
Interpretation: The organic products formed in given reaction are to be drawn.
Concept introduction: Decarboxylation occurs in carboxylic acids.[a1] It involves a cleavage of alpha carbon with loss of carbon dioxide along with heating.
Answer to Problem 23.50P
The organic product formed in given reaction is,
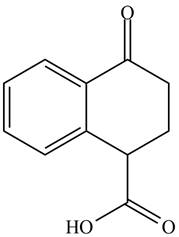
Figure 1
Explanation of Solution
Decarboxylation occurs when a carboxyl group is attached to alpha carbon of another carbonyl group. It removes the carboxylic group from the alpha carbon of the carbonyl group. The corresponding chemical reaction is shown below.
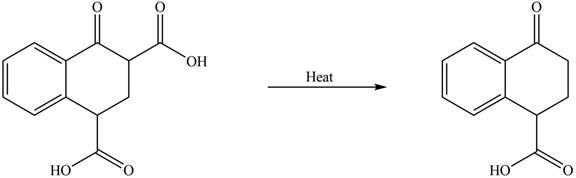
Figure 2
Hence, the organic product formed in given reaction is
The organic product formed in given reaction is shown in Figure 1.
(b)
Interpretation: The organic products formed in given reaction are to be drawn.
Concept introduction: Lithium diisopropylamide (LDA) is a sterically hindered strong base. In an unsymmetrical ketone, it abstracts hydrogen from least substituted carbon to form kinetic product.
Answer to Problem 23.50P
The organic product formed in given reaction is,
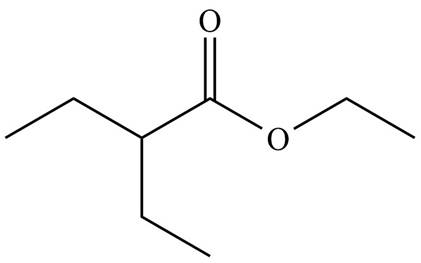
Figure 3
Explanation of Solution
LDA abstracts a proton from the alpha carbon of ethylbutanoate to form enolate. This enolate ion acts as a nucleophile and attacks on electrophilic centre of iodoethane and forms
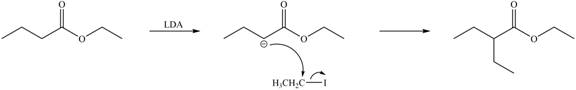
Figure 4
Hence, the organic product formed in given reaction is
The organic product formed in given reaction is shown in Figure 3.
(c)
Interpretation: The organic products formed in given reaction are to be drawn.
Concept introduction: Alkyl amines are formed by the reaction of alkyl halides with amines. This reaction is known as alkylation of amines.
Answer to Problem 23.50P
The organic product formed in given reaction is,
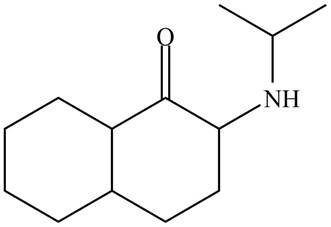
Figure 5
Explanation of Solution
In the first step,
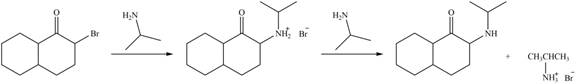
Figure 6
Hence, the organic product formed in given reaction is
The organic product formed in given reaction is shown in Figure 5.
(d)
Interpretation: The organic products formed in given reaction are to be drawn.
Concept introduction: Lithium diisopropylamide (LDA) is a sterically hindered strong base. In an unsymmetrical ketone, it abstracts hydrogen from least substituted carbon to form kinetic product.
Answer to Problem 23.50P
The organic product formed in given reaction is,
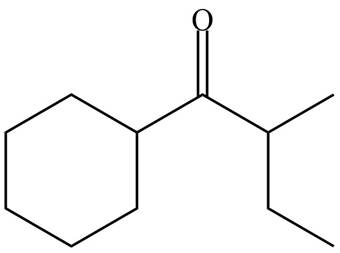
Figure 7
Explanation of Solution
LDA abstracts a proton from the less hindered alpha carbon of
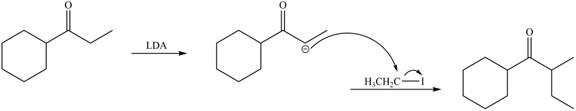
Figure 8
Hence, the organic product formed in given reaction is
The organic product formed in given reaction is shown in Figure 7.
(e)
Interpretation: The organic products formed in given reaction are to be drawn.
Concept introduction: Monobromo product is formed by the reaction of carbonyl compounds with bromine in the presence of acetic acid and further treatment of monobromo with base yield alkenes.
Answer to Problem 23.50P
The organic product formed in given reaction is,
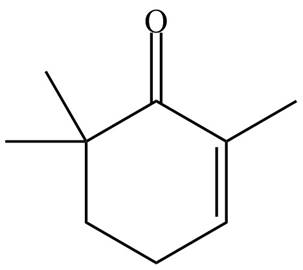
Figure 9
Explanation of Solution
Monobromo product is formed by the reaction of
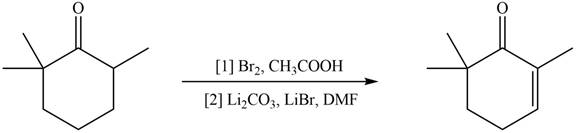
Figure 10
Hence, the organic product formed in given reaction is
The organic product formed in given reaction is shown in Figure 9.
(f)
Interpretation: The organic products formed in given reaction are to be drawn.
Concept introduction: Haloform is produced by the halogenation of a methyl ketone in the presence of a base. This reaction is also known as haloform reaction.
Answer to Problem 23.50P
The organic product formed in given reaction is,
Figure 11
Explanation of Solution
The enolate ion reacts with iodine and form
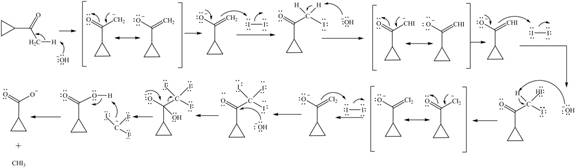
Figure 12
The organic product formed in given reaction is shown in Figure 11.
(g)
Interpretation: The organic products formed in given reaction are to be drawn.
Concept introduction: Sodium hydride is a strong base. It abstracts a proton and forms carbanion. This carbanion acts as a nucleophile and attacks on the electrophilic centre of alkyl halide.
Answer to Problem 23.50P
The organic product formed in given reaction is,
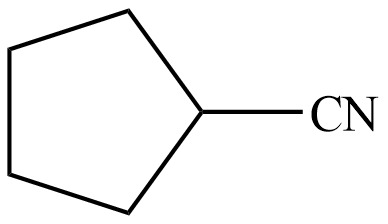
Figure 13
Explanation of Solution
Sodium hydride abstracts a proton from the carbon which is attached to cyanide and forms secondary carbanion. The next step is intra molecular cyclization. Now this carbanion acts as nucleophile and attack to the carbon (electrophile) which is directly attach to the electronegative atom i.e. chlorine. The corresponding chemical reaction of given organic compound with a strong base is shown below
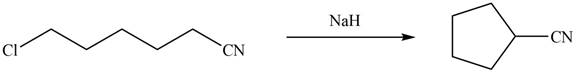
Figure 14
Hence, the organic product formed in given reaction is cyclopentanecarbonitrile.
The organic product formed in given reaction is shown in Figure 13.
(h)
Interpretation: The organic products formed in given reaction are to be drawn.
Concept introduction: Bromination is a type of radical substitution reaction. It is highly specific reaction. In this reaction, an alkyl halide is formed by the replacement of hydrogen atom from highly substituted carbon by
Answer to Problem 23.50P
The organic product formed in given reaction is,
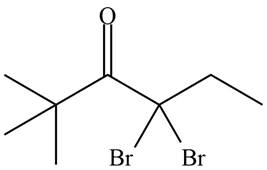
Figure 15
Explanation of Solution
An alkyl halide is formed by the replacement of hydrogen atom from highly substituted carbon by
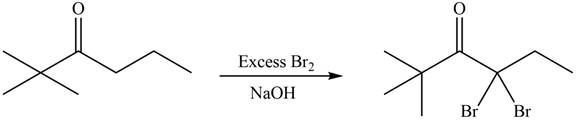
Figure 16
Hence, the organic product formed in given reaction is
The organic product formed in given reaction is shown in Figure 15.
Want to see more full solutions like this?
Chapter 23 Solutions
Organic Chemistry
- Draw the Michael Adduct and the final product of the Robinson annulation reaction. Ignore inorganic byproducts.arrow_forwardDraw the Michael adduct and final product of the Robinson annulation reaction. Ignore inorganic byproductsarrow_forwardPost Lab Questions. 1) Draw the mechanism of your Diels-Alder cycloaddition. 2) Only one isomer of product is formed in the Diels-Alder cycloaddition. Why? 3) Imagine that you used isoprene as diene - in that case you don't have to worry about assigning endo vs exo. Draw the "endo" and "exo" products of the Diels-Alder reaction between isoprene and maleic anhydride, and explain why the distinction is irrelevant here. 4) This does not hold for other dienes. Draw the exo and endo products of the reaction of cyclohexadiene with maleic anhydride. Make sure you label your answers properly as endo or exo. 100 °C Xylenes ??? 5) Calculate the process mass intensity for your specific reaction (make sure to use your actual amounts of reagent).arrow_forward
- Indicate the product(s) A, B C and D that are formed in the reaction: H + NH-NH-CH [A+B] [C+D] hydrazonesarrow_forwardHow can you prepare a 6 mL solution of 6% H2O2, if we have a bottle of 30% H2O2?arrow_forwardHow many mL of H2O2 from the 30% bottle must be collected to prepare 6 mL of 6% H2O2.arrow_forward
- Indicate the product(s) B and C that are formed in the reaction: HN' OCH HC1 B + mayoritario C minoritario OCH3arrow_forwardIndicate the product(s) that are formed in the reaction: NH-NH, OCH3 -H₂O OCH3arrow_forward21.38 Arrange the molecules in each set in order of increasing acidity (from least acidic to most acidic). OH OH SH NH2 8 NH3 OH (b) OH OH OH (c) & & & CH3 NO2 21.39 Explain the trends in the acidity of phenol and the monofluoro derivatives of phenol. OH OH OH OH PK 10.0 PK 8.81 PK 9.28 PK 9.81arrow_forward
- identify which spectrum is for acetaminophen and which is for phenacetinarrow_forwardThe Concept of Aromaticity 21.15 State the number of 2p orbital electrons in each molecule or ion. (a) (b) (e) (f) (c) (d) (h) (i) DA (k) 21.16 Which of the molecules and ions given in Problem 21.15 are aromatic according to the Hückel criteria? Which, if planar, would be antiaromatic? 21.17 Which of the following structures are considered aromatic according to the Hückel criteria? ---0-0 (a) (b) (c) (d) (e) (h) H -H .8.0- 21.18 Which of the molecules and ions from Problem 21.17 have electrons donated by a heteroatom?arrow_forward1. Show the steps necessary to make 2-methyl-4-nonene using a Wittig reaction. Start with triphenylphosphine and an alkyl halide. After that you may use any other organic or inorganic reagents. 2. Write in the product of this reaction: CH3 CH₂ (C6H5)₂CuLi H₂O+arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
