
(a)
Interpretation: The product that is formed by the reaction of the given starting material with LDA in the presence of THF solution at
Concept introduction: The replacement or substitution of one
The bulky base like LDA always abstracts the proton from the side of less substituted
Answer to Problem 23.7P
The product that is formed by the reaction of the given starting material with LDA in the presence of THF solution at
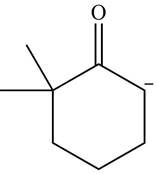
Explanation of Solution
The product that is formed by the reaction of the given starting material with LDA in THF solution at
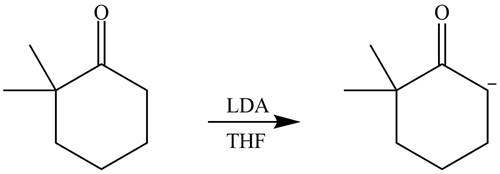
Figure 1
In this reaction,
(b)
Interpretation: The product that is formed by the reaction of the given starting material with LDA in the presence of THF solution at
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The nucleophilic reaction that consists of bimolecular as well as bond-making and bond-breaking steps is termed as
The bulky base like LDA always abstracts the proton from the side of less substituted
Answer to Problem 23.7P
The product that is formed by the reaction of the given starting material with LDA in the presence of THF solution at
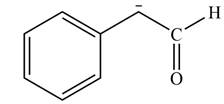
Explanation of Solution
The product that is formed by the reaction of the given starting material with LDA in THF solution at
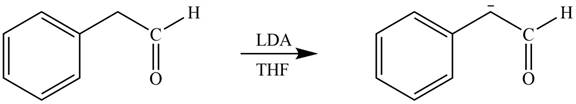
Figure 2
In this reaction,
(c)
Interpretation: The product that is formed by the reaction of the given starting material with LDA in the presence of THF solution at
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The nucleophilic reaction that consists of bimolecular as well as bond-making and bond-breaking steps is termed as
The bulky base like LDA always abstracts the proton from the side of less substituted
Answer to Problem 23.7P
The product that is formed by the reaction of the given starting material with LDA in the presence of THF solution at
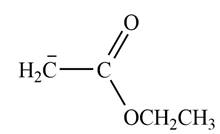
Explanation of Solution
The product that is formed by the reaction of the given starting material with LDA in THF solution at
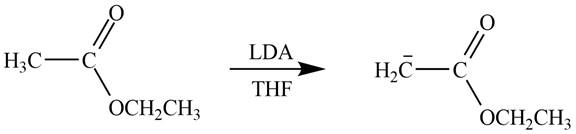
Figure 3
In this reaction, ethyl acetate is treated with LDA in the presence of THF solution to form an enolate ion. The strong base, LDA abstracts a proton from the less substituted carbon atom of the compound.
(d)
Interpretation: The product that is formed by the reaction of the given starting material with LDA in the presence of THF solution at
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The nucleophilic reaction that consists of bimolecular as well as bond-making and bond-breaking steps is termed as
The bulky base like LDA always abstracts the proton from the side of less substituted
Answer to Problem 23.7P
The product that is formed by the reaction of the given starting material with LDA in the presence of THF solution at
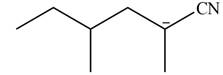
Explanation of Solution
The product that is formed by the reaction of the given starting material with LDA in THF solution at
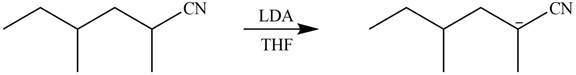
Figure 4
In this reaction,
(a) The product that is formed by the reaction of the given starting material with LDA in the presence of THF solution at
(b) The product that is formed by the reaction of the given starting material with LDA in the presence of THF solution at
(c) The product that is formed by the reaction of the given starting material with LDA in the presence of THF solution at
(d) The product that is formed by the reaction of the given starting material with LDA in the presence of THF solution at
Want to see more full solutions like this?
Chapter 23 Solutions
Organic Chemistry
- please explain this in simple termsarrow_forwardK Most Reactive Na (3 pts) Can the metal activity series (shown on the right) or a standard reduction potential table explain why potassium metal can be prepared from the reaction of molten KCI and Na metal but sodium metal is not prepared from the reaction of molten NaCl and K metal? Show how (not). Ca Mg Al с Zn Fe Sn Pb H Cu Ag Au Least Reactivearrow_forward(2 pts) Why is O2 more stable as a diatomic molecule than S2?arrow_forward
- Draw the Lewis structure for the polyatomic phosphite (PO¾³¯) a anion. Be sure to include all resonance structures that satisfy the octet rule. C I A [ ]¯arrow_forwardDecide whether these proposed Lewis structures are reasonable. proposed Lewis structure Is the proposed Lewis structure reasonable? Yes. :0: Cl C C1: 0=0: : 0 : : 0 : H C N No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* ☐ Yes. No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. ☐ No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* | * If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "0,0".arrow_forwardDraw the Lewis structure for the polyatomic trisulfide anion. Be sure to include all resonance structures that satisfy the octet rule. с [ ] - Garrow_forward
- 1. Calculate the accurate monoisotopic mass (using all 1H, 12C, 14N, 160 and 35CI) for your product using the table in your lab manual. Don't include the Cl, since you should only have [M+H]*. Compare this to the value you see on the LC-MS printout. How much different are they? 2. There are four isotopic peaks for the [M+H]* ion at m/z 240, 241, 242 and 243. For one point of extra credit, explain what each of these is and why they are present. 3. There is a fragment ion at m/z 184. For one point of extra credit, identify this fragment and confirm by calculating the accurate monoisotopic mass. 4. The UV spectrum is also at the bottom of your printout. For one point of extra credit, look up the UV spectrum of bupropion on Google Images and compare to your spectrum. Do they match? Cite your source. 5. For most of you, there will be a second chromatographic peak whose m/z is 74 (to a round number). For one point of extra credit, see if you can identify this molecule as well and confirm by…arrow_forwardPlease draw, not just describe!arrow_forwardcan you draw each step on a piece of a paper please this is very confusing to mearrow_forward
- > Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? esc ? A O O •If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. • If your answer is no, check the box under the drawing area instead. olo 18 Ar Explanation Check BB Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibilityarrow_forwardName the structurearrow_forward> For each pair of substrates below, choose the one that will react faster in a substitution reaction, assuming that: 1. the rate of substitution doesn't depend on nucleophile concentration and 2. the products are a roughly 50/50 mixture of enantiomers. Substrate A Substrate B Faster Rate X CI (Choose one) (Choose one) CI Br Explanation Check Br (Choose one) C 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy A F10arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





