
(a)
Interpretation: The product that is formed by the reaction of the given compound with LDA in the presence of THF solution at low temperature, followed by
Concept introduction: The replacement or substitution of one
The bulky base like LDA always abstracts the proton from the side of less substituted
Answer to Problem 23.15P
The product that is formed by the reaction of the given compound with LDA in the presence of THF solution at low temperature, followed by
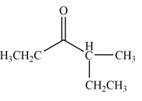
Explanation of Solution
The product that is formed by the reaction of the given compound with LDA in the presence of THF solution at low temperature, followed by
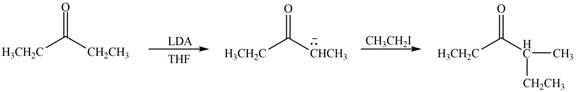
Figure 1
In this reaction, the given compound is treated with LDA in the presence of THF solution to form an enolate ion. The strong base, LDA abstracts a proton from the less substituted carbon atom of the compound. Then methyl iodide reacts with the enolte ion to form the desired product.
The product that is formed by the reaction of the given compound with LDA in the presence of THF solution at low temperature, followed by
(b)
Interpretation: The product that is formed by the reaction of the given compound with LDA in the presence of THF solution at low temperature, followed by
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The nucleophilic reaction that consists of bimolecular as well as bond-making and bond-breaking steps is termed as
The bulky base like LDA always abstracts the proton from the side of less substituted
Answer to Problem 23.15P
The product that is formed by the reaction of the given compound with LDA in the presence of THF solution at low temperature, followed by
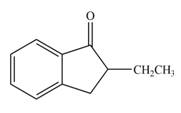
Explanation of Solution
The product that is formed by the reaction of the given compound with LDA in the presence of THF solution at low temperature, followed by
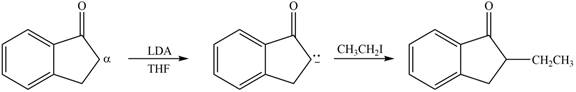
Figure 2
In this reaction, the given compound is treated with LDA in the presence of THF solution to form an enolate ion. The strong base, LDA abstracts a proton from the less substituted carbon atom or
The product that is formed by the reaction of the given compound with LDA in the presence of THF solution at low temperature, followed by
(c)
Interpretation: The product that is formed by the reaction of the given compound with LDA in the presence of THF solution at low temperature, followed by
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The nucleophilic reaction that consists of bimolecular as well as bond-making and bond-breaking steps is termed as
The bulky base like LDA always abstracts the proton from the side of less substituted
Answer to Problem 23.15P
The product that is formed by the reaction of the given compound with LDA in the presence of THF solution at low temperature, followed by
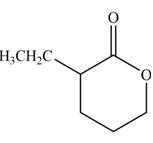
Explanation of Solution
The product that is formed by the reaction of the given compound with LDA in the presence of THF solution at low temperature, followed by
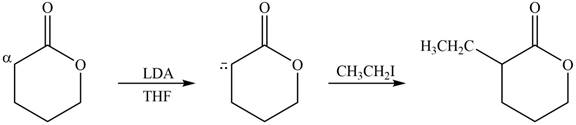
Figure 3
In this reaction, the given compound is treated with LDA in the presence of THF solution to form an enolate ion. The strong base, LDA abstracts a proton from the less substituted carbon atom or
The product that is formed by the reaction of the given compound with LDA in the presence of THF solution at low temperature, followed by
(d)
Interpretation: The product that is formed by the reaction of the given compound with LDA in the presence of THF solution at low temperature, followed by
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The nucleophilic reaction that consists of bimolecular as well as bond-making and bond-breaking steps is termed as
The bulky base like LDA always abstracts the proton from the side of less substituted
Answer to Problem 23.15P
The product that is formed by the reaction of the given compound with LDA in the presence of THF solution at low temperature, followed by
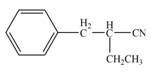
Explanation of Solution
The product that is formed by the reaction of the given compound with LDA in the presence of THF solution at low temperature, followed by
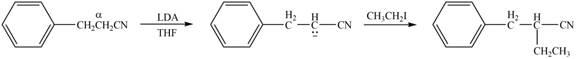
Figure 4
In this reaction, the given compound is treated with LDA in the presence of THF solution to form an enolate ion. The strong base, LDA abstracts a proton from the less substituted carbon atom or
The product that is formed by the reaction of the given compound with LDA in the presence of THF solution at low temperature, followed by
Want to see more full solutions like this?
Chapter 23 Solutions
ORGANIC CHEMISTRY
- Please predict the products for each of the following reactions: 1.03 2. H₂O NaNH, 1. n-BuLi 2. Mel A H₂ 10 9 0 H2SO4, H₂O HgSO4 Pd or Pt (catalyst) B 9 2 n-BuLi ♡ D2 (deuterium) Lindlar's Catalyst 1. NaNH2 2. EtBr Na, ND3 (deuterium) 2. H₂O2, NaOH 1. (Sia)2BH с Darrow_forwardin the scope of ontario SCH4U grade 12 course, please show ALL workarrow_forwardIs the chemical reaction CuCl42-(green) + 4H2O <==> Cu(H2O)42+(blue) + 4Cl- exothermic or endothermic?arrow_forward
- If we react tetraethoxypropane with hydrazine, what is the product obtained (explain its formula). State the reason why the corresponding dialdehyde is not used.arrow_forwarddrawing, no aiarrow_forwardIf CH3COCH2CH(OCH3)2 (4,4-dimethoxy-2-butanone) and hydrazine react, two isomeric products are formed. State their structure and which will be the majority.arrow_forward
- + Reset Provide the correct IUPAC name for the compound shown here. 4-methylhept-2-ene (Z)- (E)- 1-6-5-2-3-4- cyclo iso tert- sec- di tri hept hex oct meth eth pent ane yne ene ylarrow_forward+ Provide the correct IUPAC name for the compound shown here. Reset H3C H H C CH3 CH-CH3 1-3-methylpent ene trans- cis- 5-6-3-1-2-4- tert- tri sec- di cyclo iso but pent hex meth prop eth yl ane ene yne ☑arrow_forwarddrawing, no aiarrow_forward

 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

