
(a)
Interpretation: The
Concept introduction: The Malonic ester synthesis is a method which is used to convert diethyl malonate into a carboxylic acid.
Answer to Problem 23.43P
The alkyl halide which is needed to prepare given carboxylic acid using the malonic ester synthesis is
Explanation of Solution
The given carboxylic acid is,
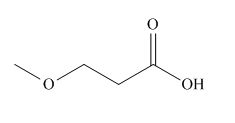
Figure 1
All alkyl groups on the alpha carbon will be obtained from alkyl halides and the remaining molecule is obtained from ethyl acetoacetate. Thus, for the required alkyl halide, the location of alpha carbon is shown below.
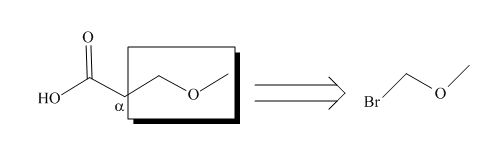
Figure 2
Hence, the alkyl halide which is needed to prepare given carboxylic acid using the malonic ester synthesis is
The reaction of diethylmalonate with
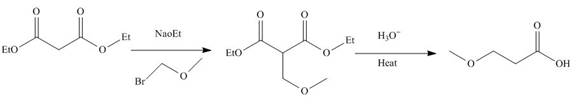
Figure 3
The mechanism involved in the given reaction as follows,
Base abstracts the proton from the alpha carbon and enolate ion reacts with
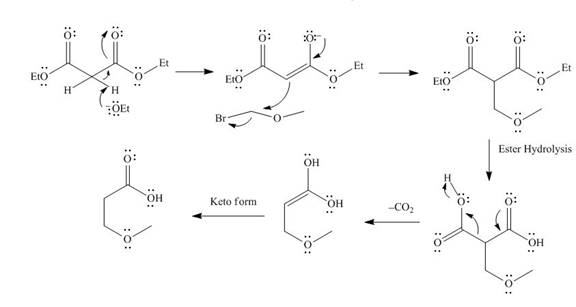
Figure 4
The alkyl halide which is needed to prepare given carboxylic acid using the malonic ester synthesis is
(b)
Interpretation: The alkyl halide which is needed to prepare given carboxylic acid using the malonic ester synthesis is to be predicted.
Concept introduction: The Malonic ester synthesis is a method which is used to convert diethyl malonate into a carboxylic acid.
Answer to Problem 23.43P
The alkyl halide which is needed to prepare given carboxylic acid using the malonic ester synthesis is
Explanation of Solution
The given carboxylic acid is,
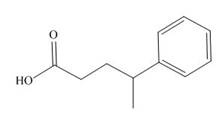
Figure 5
All alkyl groups on the alpha carbon will be obtained from alkyl halides and the remaining molecule is obtained from ethyl acetoacetate. Thus, for the required alkyl halide, the location of alpha carbon is shown below.
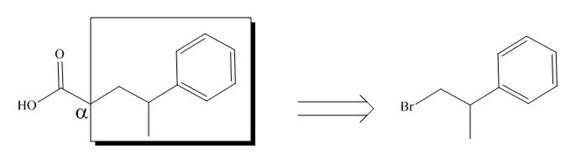
Figure 6
Hence, the alkyl halide which is needed to prepare given carboxylic acid using the malonic ester synthesis is
The reaction of diethylmalonate with
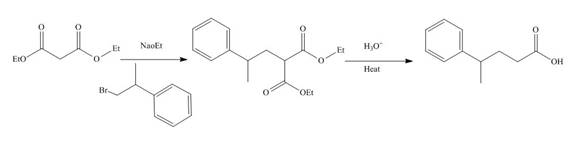
Figure 7
The mechanism involved in the given reaction as follows,
Base abstracts the proton from the alpha carbon and enolate reacts with
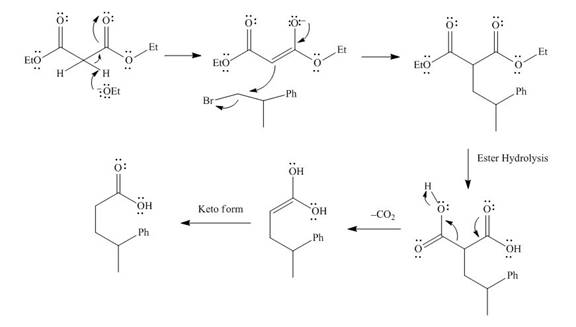
Figure 8
The alkyl halide which is needed to prepare given carboxylic acid using the malonic ester synthesis is
(c)
Interpretation: The alkyl halide which is needed to prepare given carboxylic acid using the malonic ester synthesis is to be predicted.
Concept introduction: The Malonic ester synthesis is a method which is used to convert diethyl malonate into a carboxylic acid.
Answer to Problem 23.43P
The alkyl halide which is needed to prepare given carboxylic acid using the malonic ester synthesis is
Explanation of Solution
The given carboxylic acid is,
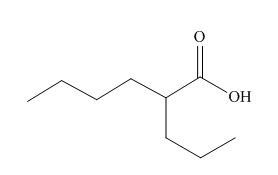
Figure 9
All alkyl groups on the alpha carbon will be obtained from alkyl halides and the remaining molecule is obtained from ethyl acetoacetate. Thus, for the required alkyl halide, the location of alpha carbon is shown below.
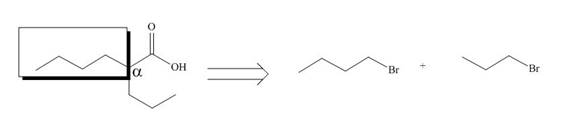
Figure 10
Hence, the alkyl halide which is needed to prepare given carboxylic acid using the malonic ester synthesis is
The reaction of diethylmalonate with
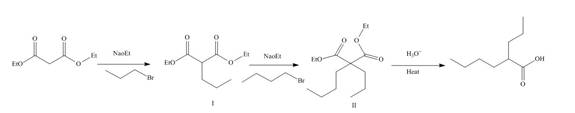
Figure 11
The mechanism involved in the given reaction as follows,
Base abstracts the proton from the alpha carbon and enolate reacts with
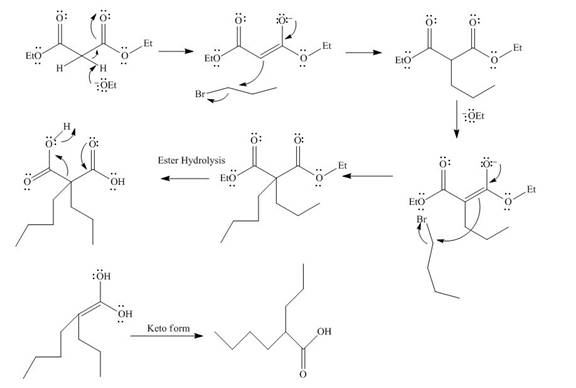
Figure 12
The alkyl halide which is needed to prepare given carboxylic acid using the malonic ester synthesis is
Want to see more full solutions like this?
Chapter 23 Solutions
ORGANIC CHEMISTRY
- Write all of Me Possible Products For each Of the Following reactions. In each case identity all pains of enantiomers, all digsterzoners and all Meso compounds 9. 11-60 11-0-11 V-G Η Η H ~ C-11 +HB+ - 1 H b. पन्ना 171-0-11 H-C-H Н C-C=c-call +HBr Perendez ==arrow_forwardHow can i draw the mechanisms for this molecule?arrow_forwarda. Discuss and explain he difference IN Stability between the Chai and Boat Гольцу от судомехане b. For the Following Molecule draw both possible Clain conformations and explain which one is more stable and for what Reason. H. CH₂ CH₂ H "Harrow_forward
- Please provide the mechanism for this reacitonarrow_forwardQuestion 5: Name the following compound in two ways using side chain and using prefix amine (Common name and IUPAC name both) CH3NH2 CH3CH2NHCH3 CH₂CH₂N(CH3)2 Draw the structure of diethyl methyl amine Question 6. Write the balanced combustion reaction for: a. Hexane b. Propyne c. 2-pentene Question 7: Write the following electrophilic substitution reactions of benzene: Hint: Use notes if you get confused a. Halogenation reaction: b. Nitration reaction : c. Sulphonation reaction: d. Alkylation reaction: e. Aceylation reaction:arrow_forwardQuestion 4. Name the following structures ○ CH3-C-N-H H CH3CH2-C-N-H H CH3CH2-C-N-CH3 Harrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
