
Concept explainers
Draw the enol or keto tautomer(s) of each compound.
a. 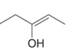 c.
c. 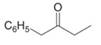 e.
e. 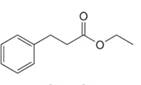
b. 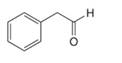 d.
d. 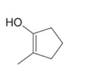 f.
f. 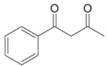 [Draw mono enol
[Draw mono enol
tautomers only]
(a)
Interpretation: The enol or keto tautomer of the given compound is to be drawn.
Concept introduction: The chemical equilibrium that exists between a keto form of a compound and an enol form of the same compound is known as keto-enol tautomerism. Tautomers refer to these keto and enol forms. The keto-enol tautomerism takes place only if the acidic hydrogen is present at the
Answer to Problem 23.1P
The enol or keto tautomer of the given compound is shown below.
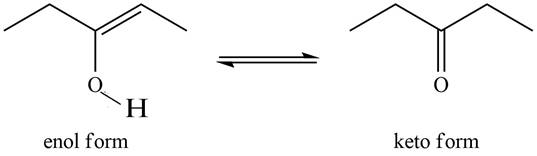
Explanation of Solution
The enol or keto tautomer of the given compound is shown as,
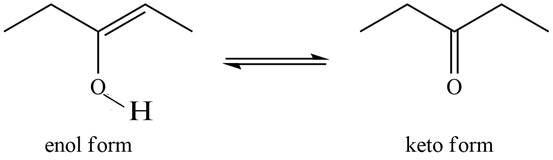
Figure 1
The keto form of the given enol form of a compound is shown appropriately because it contains the acidic hydrogen is present at the
The enol or keto tautomer of the given compound is shown in Figure 1.
(b)
Interpretation: The enol or keto tautomer of the given compound is to be drawn.
Concept introduction: The chemical equilibrium that exists between a keto form of a compound and an enol form of the same compound is known as keto-enol tautomerism. Tautomers refer to these keto and enol forms. The keto-enol tautomerism takes place only if the acidic hydrogen is present at the
Answer to Problem 23.1P
The enol or keto tautomer of the given compound is shown below.
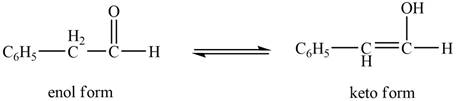
Explanation of Solution
The enol or keto tautomer of the given compound is shown as,
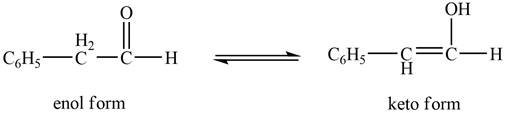
Figure 2
The enol form of the given keto form of a compound is shown appropriately because it contains the acidic hydrogen is present at the
The enol or keto tautomer of the given compound is shown in Figure 2.
(c)
Interpretation: The enol or keto tautomer of the given compound is to be drawn.
Concept introduction: The chemical equilibrium that exists between a keto form of a compound and an enol form of the same compound is known as keto-enol tautomerism. Tautomers refer to these keto and enol forms. The keto-enol tautomerism takes place only if the acidic hydrogen is present at the
Answer to Problem 23.1P
The enol or keto tautomer of the given compound is shown below.
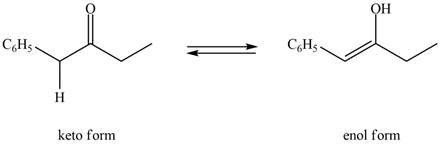
Explanation of Solution
The enol or keto tautomer of the given compound is shown as,
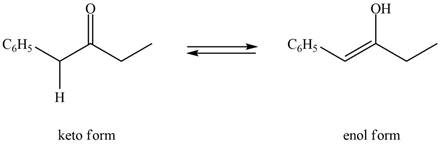
Figure 3
The enol form of the given keto form of a compound is shown appropriately because it contains the acidic hydrogen is present at the
The enol or keto tautomer of the given compound is shown in Figure 3.
(d)
Interpretation: The enol or keto tautomer of the given compound is to be drawn.
Concept introduction: The chemical equilibrium that exists between a keto form of a compound and an enol form of the same compound is known as keto-enol tautomerism. Tautomers refer to these keto and enol forms. The keto-enol tautomerism takes place only if the acidic hydrogen is present at the
Answer to Problem 23.1P
The enol or keto tautomer of the given compound is shown below.
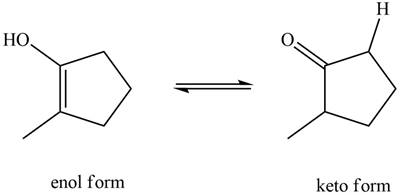
Explanation of Solution
The enol or keto tautomer of the given compound is shown as,

Figure 4
The keto form of the given enol form of a compound is shown appropriately because it contains the acidic hydrogen is present at the
The enol or keto tautomer of the given compound is shown in Figure 4.
(e)
Interpretation: The enol or keto tautomer of the given compound is to be drawn.
Concept introduction: The chemical equilibrium that exists between a keto form of a compound and an enol form of the same compound is known as keto-enol tautomerism. Tautomers refer to these keto and enol forms. The keto-enol tautomerism takes place only if the acidic hydrogen is present at the
Answer to Problem 23.1P
The enol or keto tautomer of the given compound is shown below.
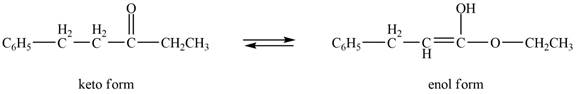
Explanation of Solution
The enol or keto tautomer of the given compound is shown as,
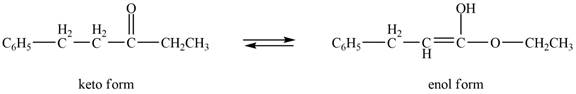
Figure 5
The enol form of the given keto form of a compound is shown appropriately because it contains the acidic hydrogen is present at the
The enol or keto tautomer of the given compound is shown in Figure 5.
(f)
Interpretation: The enol or keto tautomer of the given compound is to be drawn.
Concept introduction: The chemical equilibrium that exists between a keto form of a compound and an enol form of the same compound is known as keto-enol tautomerism. Tautomers refer to these keto and enol forms. The keto-enol tautomerism takes place only if the acidic hydrogen is present at the
Answer to Problem 23.1P
The enol or keto tautomer of the given compound is shown below.
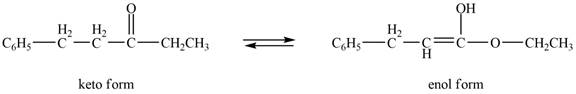
Explanation of Solution
The enol or keto tautomer of the given compound is shown as,

Figure 6
The enol form of the given keto form of a compound is shown appropriately because it contains the acidic hydrogen is present at the
The enol or keto tautomer of the given compound is shown in Figure 6.
Want to see more full solutions like this?
Chapter 23 Solutions
ORGANIC CHEMISTRY
- Draw the mechanism to make the alcohol 2-hexanol. Draw the Mechanism to make the alcohol 1-hexanol.arrow_forwardDraw the mechanism for the formation of diol by starting with 1-pentanal in... basic conditions then acidic conditions then draw the mechanism for the formation of a carboxylic acid from your product.arrow_forwardIdentify each chiral carbon as either R or S. Identify the overall carbohydrates as L or Darrow_forward
- Ethers can be formed via acid-catalyzed acetal formation. Draw the mechanism for the molecule below and ethanol.arrow_forwardHOCH, H HO CH-OH OH H OH 11 CH₂OH F II OH H H 0 + H OHarrow_forwardDraw the mechanism for the formation of diol by starting with one pen and all in... basic conditions then acidic conditions then draw the mechanism for the formation of a carboxylic acid from your product.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





