
Organic Chemistry-Package(Custom)
4th Edition
ISBN: 9781259141089
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 22, Problem 22.79P
How can IR spectroscopy be used to distinguish between each pair of isomers?
a. 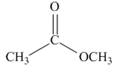 and
and 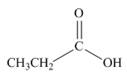 c.
c. 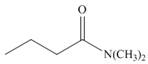 and
and 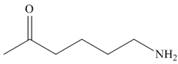
b. 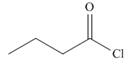 and
and 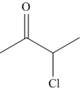 d.
d. 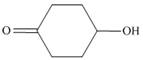 and
and 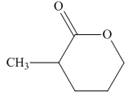
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
This is a synthesis question. Why is this method wrong or worse than the "correct" method? You could do it thiss way, couldn't you?
Try: Draw the best Lewis structure showing all non-bonding electrons and all formal charges if
any:
(CH3)3CCNO
NCO-
HN3
[CH3OH2]*
What are the major products of the following reaction?
Draw all the major products. If there are no major products, then there is no reaction that will take place. Use wedge and dash bonds when necessary.
Chapter 22 Solutions
Organic Chemistry-Package(Custom)
Ch. 22 - Prob. 22.1PCh. 22 - Draw the three possible resonance structures for...Ch. 22 - Prob. 22.3PCh. 22 - Give an IUPAC or common name for each compound.Ch. 22 - Problem 22.5 Draw the structure corresponding to...Ch. 22 - Prob. 22.6PCh. 22 - How would the compounds in each pair differ in...Ch. 22 - Problem 22.8 Deduce the structures of compounds ...Ch. 22 - Prob. 22.9PCh. 22 - Rank the compounds in each group in order of...
Ch. 22 - Prob. 22.11PCh. 22 - Prob. 22.12PCh. 22 - Prob. 22.13PCh. 22 - Prob. 22.14PCh. 22 - Problem 22.15 Draw the products of each...Ch. 22 - Problem 22.16 Draw the products of each reaction.
...Ch. 22 - Prob. 22.17PCh. 22 - Problem 22.18 Draw a stepwise mechanism for the...Ch. 22 - Prob. 22.19PCh. 22 - Problem 22.20 Fenofibrate is a...Ch. 22 - Problem 22.21 What product is formed when the...Ch. 22 - How would you synthesize olestra from sucrose?
Ch. 22 - What is the composition of the soap prepared by...Ch. 22 - Problem 22.24 Draw a stepwise mechanism for the...Ch. 22 - Prob. 22.25PCh. 22 - Problem 22.26 Some penicillins cannot be...Ch. 22 - Prob. 22.27PCh. 22 - Prob. 22.28PCh. 22 - Prob. 22.29PCh. 22 - Problem 22.30 Glucosamine is a dietry supplement...Ch. 22 - Draw the products of each reaction. a. c. b.Ch. 22 - Draw a tautomer of each compound.Ch. 22 - Draw the product of each reaction. a.b.Ch. 22 - Draw the product of each reaction. a. b.Ch. 22 - Prob. 22.35PCh. 22 - Problem 22.36 Outline two different ways that can...Ch. 22 - 22.37 Rank the following compounds in order of...Ch. 22 - Prob. 22.38PCh. 22 - Prob. 22.39PCh. 22 - Prob. 22.40PCh. 22 - Give thestructure corresponding to each name. a....Ch. 22 - Prob. 22.42PCh. 22 - Prob. 22.43PCh. 22 - 22.43 Explain why is a stronger acid and a weaker...Ch. 22 - Draw the product formed when pentanoyl chloride...Ch. 22 - Draw the product formed when pentanoic anhydride...Ch. 22 - Draw the product formed when phenylacetic acid is...Ch. 22 - Prob. 22.48PCh. 22 - Prob. 22.49PCh. 22 - Draw the product formed when phenylacetonitrile ...Ch. 22 - Prob. 22.51PCh. 22 - Prob. 22.52PCh. 22 - Prob. 22.53PCh. 22 - Prob. 22.54PCh. 22 - Prob. 22.55PCh. 22 - Prob. 22.56PCh. 22 - Draw a stepwise mechanism for each reaction. a. b.Ch. 22 - When acetic acid CH3COOH is treated with a trace...Ch. 22 - Prob. 22.59PCh. 22 - Prob. 22.60PCh. 22 - Prob. 22.61PCh. 22 - Prob. 22.62PCh. 22 - Prob. 22.63PCh. 22 - Draw a stepwise mechanism for the following...Ch. 22 - 22.63 Acid-catalyzed hydrolysis of forms compound...Ch. 22 - Prob. 22.66PCh. 22 - Devise a synthesis of each compound using...Ch. 22 - Convert 1-bromohexane (CH3CH2CH2CH2CH2CH2Br) into...Ch. 22 - Prob. 22.69PCh. 22 - Prob. 22.70PCh. 22 - Prob. 22.71PCh. 22 - Prob. 22.72PCh. 22 - Prob. 22.73PCh. 22 - Prob. 22.74PCh. 22 - Devise a synthesis of each labeled compound using...Ch. 22 - 22.70 What polyester or poly amide can be prepared...Ch. 22 - 22.71 What two monomers are needed to prepare each...Ch. 22 - Prob. 22.78PCh. 22 - How can IR spectroscopy be used to distinguish...Ch. 22 - Rank the compounds in each group in order of...Ch. 22 - 22.75 Identify the structures of each compound...Ch. 22 - 22.76 Identify the structures of A and B, isomers...Ch. 22 - Prob. 22.83PCh. 22 - 22.78 Identify the structure of compound C...Ch. 22 - 22.79 Identify the structures of D and E, isomers...Ch. 22 - 22.80 With reference to amides A and B, the...Ch. 22 - Prob. 22.87PCh. 22 - Prob. 22.88PCh. 22 - Prob. 22.89PCh. 22 - Draw a stepwise mechanism for the following...Ch. 22 - Draw a stepwise mechanism for the following...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- IX) By writing the appropriate electron configurations and orbital box diagrams briefly EXPLAIN in your own words each one of the following questions: a) The bond length of the Br2 molecule is 2.28 Å, while the bond length of the compound KBr is 3.34 Å. The radius of K✶ is 1.52 Å. Determine the atomic radius in Å of the bromine atom and of the bromide ion. Br = Br b) Explain why there is a large difference in the atomic sizes or radius of the two (Br and Br). Tarrow_forwardWhen 15.00 mL of 3.00 M NaOH was mixed in a calorimeter with 12.80 mL of 3.00 M HCl, both initially at room temperature (22.00 C), the temperature increased to 29.30 C. The resultant salt solution had a mass of 27.80 g and a specific heat capacity of 3.74 J/Kg. What is heat capacity of the calorimeter (in J/C)? Note: The molar enthalpy of neutralization per mole of HCl is -55.84 kJ/mol.arrow_forwardWhen 15.00 mL of 3.00 M NaOH was mixed in a calorimeter with 12.80 mL of 3.00 M HCl, both initially at room temperature (22.00 C), the temperature increased to 29.30 C. The resultant salt solution had a mass of 27.80 g and a specific heat capacity of 3.74 J/Kg. What is heat capacity of the calorimeter (in J/C)? Note: The molar enthalpy of neutralization per mole of HCl is -55.84 kJ/mol. Which experimental number must be initialled by the Lab TA for the first run of Part 1 of the experiment? a) the heat capacity of the calorimeter b) Mass of sample c) Ti d) The molarity of the HCl e) Tfarrow_forward
- Predict products for the Following organic rxn/s by writing the structurels of the correct products. Write above the line provided" your answer D2 ①CH3(CH2) 5 CH3 + D₂ (adequate)" + 2 mited) 19 Spark Spark por every item. 4 CH 3 11 3 CH 3 (CH2) 4 C-H + CH3OH CH2 CH3 + CH3 CH2OH 0 CH3 fou + KMnDy→ C43 + 2 KMn Dy→→ C-OH ") 0 C-OH 1110 (4.) 9+3 =C CH3 + HNO 3 0 + Heat> + CH3 C-OH + Heat CH2CH3 - 3 2 + D Heat H 3 CH 3 CH₂ CH₂ C = CH + 2 H₂ → 2 2arrow_forwardWhen 15.00 mL of 3.00 M NaOH was mixed in a calorimeter with 12.80 mL of 3.00 M HCl, both initially at room temperature (22.00 C), the temperature increased to 29.30 C. The resultant salt solution had a mass of 27.80 g and a specific heat capacity of 3.74 J/Kg. What is heat capacity of the calorimeter (in J/C)? Note: The molar enthalpy of neutralization per mole of HCl is -55.84 kJ/mol.arrow_forwardQ6: Using acetic acid as the acid, write the balanced chemical equation for the protonation of the two bases shown (on the -NH2). Include curved arrows to show the mechanism. O₂N- O₂N. -NH2 -NH2 a) Which of the two Bronsted bases above is the stronger base? Why? b) Identify the conjugate acids and conjugate bases for the reactants. c) Identify the Lewis acids and bases in the reactions.arrow_forward
- Q5: For the two reactions below: a) Use curved electron-pushing arrows to show the mechanism for the reaction in the forward direction. Redraw the compounds to explicitly illustrate all bonds that are broken and all bonds that are formed. b) Label Bronsted acids and bases in the left side of the reactions. c) For reaction A, which anionic species is the weakest base? Which neutral compound is the stronger acid? Is the forward or reverse reaction favored? d) Label Lewis acids and bases, nucleophiles and electrophiles in the left side of the reactions. A. 용 CH3OH я хон CH3O OH B. HBr CH3ONa NaBr CH3OHarrow_forwardpotential energy Br b) Translate the Newman projection below to its wedge-and-dash drawing. F H. OH CH3 CI c) Isopentane (2-methylbutane) is a compound containing a branched carbon chain. Draw a Newman projection of six conformations about the C2-C3 bond of isopentane. On the curve of potential energy versus angle of internal rotation for isopentane, label each energy maximum and minimum with one of the conformations. 0° 。 F A B D C angle of internal rotation E F 360° (=0°) JDownlarrow_forwardQ7: Identify the functional groups in these molecules a) CH 3 b) Aspirin: HO 'N' Capsaicin HO O CH3 CH 3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY