
Concept explainers
(a)
Interpretation: The products formed by the treatment of benzoic anhydride
Concept introduction: Anhydrides involve two carbonyl groups. The nucleophilic substitution reaction of anhydride involves the attack of nucleophilic at one carbonyl group, which causes the second carbonyl group to be the part of leaving group.
Answer to Problem 22.14P
The products formed by the treatment of benzoic anhydride
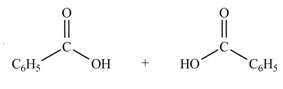
Explanation of Solution
The given treatment involves reaction of benzoic anhydride
Anhydrides involve two carbonyl groups. The nucleophilic substitution reaction of anhydride involves the attack of nucleophilic at one carbonyl group, which causes the second carbonyl group to be the part of leaving group.
Anhydrides yield two equivalents of carboxylic acids, when they are treated with
The products formed by the treatment of benzoic anhydride
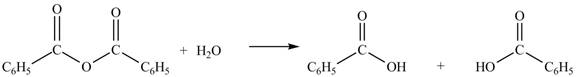
Figure 1
The products formed by the treatment of benzoic anhydride
(b)
Interpretation: The products formed by the treatment of benzoic anhydride
Concept introduction: Anhydrides involve two carbonyl groups. The nucleophilic substitution reaction of anhydride involves the attack of nucleophilic at one carbonyl group, which causes the second carbonyl group to be the part of leaving group.
Answer to Problem 22.14P
The products formed by the treatment of benzoic anhydride
Explanation of Solution
The given treatment involves reaction of benzoic anhydride
Anhydrides involve two carbonyl groups. The nucleophilic substitution reaction of anhydride involves the attack of nucleophilic at one carbonyl group, which causes the second carbonyl group to be the part of leaving group.
Anhydrides yield esters, and carboxylic acids, when they are treated with
The products formed by the treatment of benzoic anhydride
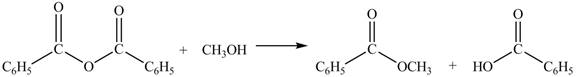
Figure 2
The products formed by the treatment of benzoic anhydride
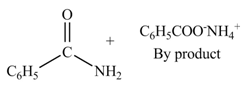
(c)
Interpretation: The products formed by the treatment of benzoic anhydride
Concept introduction: Anhydrides involve two carbonyl groups. The nucleophilic substitution reaction of anhydride involves the attack of nucleophilic at one carbonyl group, which causes the second carbonyl group to be the part of leaving group.
Answer to Problem 22.14P
The products formed by the treatment of benzoic anhydride
Explanation of Solution
The given treatment involves reaction of benzoic anhydride
Anhydrides involve two carbonyl groups. The nucleophilic substitution reaction of anhydride involves the attack of nucleophilic at one carbonyl group, which causes the second carbonyl group to be the part of leaving group.
Anhydrides yield amide and an ammonium salt of
The products formed by the treatment of benzoic anhydride
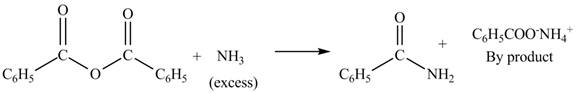
Figure 3
The products formed by the treatment of benzoic anhydride
(d)
Interpretation: The products formed by the treatment of benzoic anhydride
Concept introduction: Anhydrides involve two carbonyl groups. The nucleophilic substitution reaction of anhydride involves the attack of nucleophilic at one carbonyl group, which causes the second carbonyl group to be the part of leaving group.
Answer to Problem 22.14P
The products formed by the treatment of benzoic anhydride
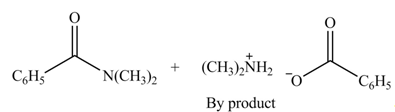
Explanation of Solution
The given treatment involves reaction of benzoic anhydride
Anhydrides involve two carbonyl groups. The nucleophilic substitution reaction of anhydride involves the attack of nucleophilic at one carbonyl group, which causes the second carbonyl group to be the part of leaving group.
Anhydrides yield amide and an ammonium salt of carboxylic acid, when they are treated with
The products formed by the treatment of benzoic anhydride
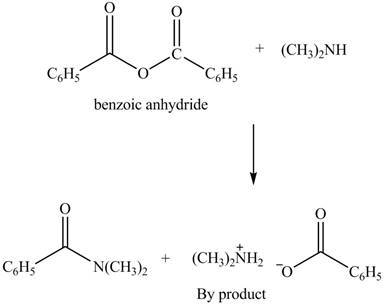
Figure 4
The products formed by the treatment of benzoic anhydride
Want to see more full solutions like this?
Chapter 22 Solutions
Organic Chemistry-Package(Custom)
- 1. Using a Model set Build a model for the following compound [CHBRIF] 2. Build another model of the mirror image of your first molecule. 3. Place the two models next to each other and take a picture which shows the differences between the two models. 4. Determine the absolute stereochemistry R or S for the two models. 5. Write or type a paragraph to Discuss the stereochemical relationship between the two models of CHBгCIF. You must provide an explanation for your conclusions also provide a description for the colors used to representarrow_forwardThe specific rotation of a sample depends upon measured angle of rotation, the density of the sample, and the pathway length of the light. True Falsearrow_forwardConsider the molecule A,B, C and D shown below, (1 x 4) Br NH2 A OH Br 边 H B C D 1. Assign the R/S configuration to each chiral center and identify by circling all the chiral centers. 2. Draw an image for the enantiomer of each of the compounds A, B, C and D.arrow_forward
- Could you crystallize one enantiomer of mandelic acid from a racemic mixture (using the typical achiral solvents found in our lab) without preparing a diastereomeric salt? Why or why not? No, because both enantiomers have the same solubility in achiral solvents. than the other. ооо Yes, because one enantiomer has a higher melting point No, because both enantiomers are liquids. Yes, because one enantiomer is more crystalline than the other.arrow_forwardIf the literature value of specific rotation for a chiral compound is -53.6°, what is the enantiomeric excess of a compound with a measured specific rotation of -40.5°?arrow_forwardThe process to determine the configuration, starts by placing the lowest priority substituent toward the back. If the substituents pointing forward decrease in priority in a clockwise order, the configuration is S. If the substituents decrease in priority in a counterclockwise order, the configuration is R. True Falsearrow_forward
- In the drawing area below, create a hemiacetal with 1 hydroxyl group, 1 methoxy group, and a total of 3 carbon atoms. Click and drag to start drawing a structure. Explanation Check Х PO 18 Ar B © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forwardPredict the product of the reaction below (3 pts). hydrazine Ph H₂NNH2 KOH Write the mechanism for the above reaction using curved arrows to show electron movements. show all intermediates in the process (7 pts).arrow_forward↓ Feedback (8/10) Draw the major product of this reaction. Ignore inorganic byproducts. Incorrect, 2 attempts remaining N H3O+ 0 × Select to Draw + V Retryarrow_forward
- 2. Calculate the branching ratio of the reaction of the methyl peroxy radical with either HO, NO 298K) (note: rate constant can be found in the tropospheric chemistry ppt CH,O,+NO-HCHO+HO, + NO, CH₂O+HO, CH₂00H +0₂ when the concentration of hydroperoxyl radical is DH01-1.5 x 10 molecules and the nitrogen oxide maxing ratio of 10 ppb when the concentration of hydroperoxyl radicalis [H0] +1.5x10 molecules cm" and the nitrogen oxide mixing ratio of 30 p Under which condition do you expect more formaldehyde to be produced and whyarrow_forwardIndicate the product of the reaction of benzene with 1-chloro-2,2-dimethylpropane in the presence of AlCl3.arrow_forwardIn what position will N-(4-methylphenyl)acetamide be nitrated and what will the compound be called.arrow_forward
