
Concept explainers
Draw the structure corresponding to each name.
a.
b. isopropyl propanoate
c. acetic formic anhydride
d. N-isobutyl-N-methylbutanamide
e.
f. o-cyanobenzoic acid
g.
h. N-ethylhexanamide
(a)
Interpretation: The structure corresponding to the given name is to be drawn.
Concept introduction: One should follow the given steps to draw the structure of a compound from its name. The first step is identification of parent name and functional group found at the end of the name. The second step is numbering of carbon skeleton in the direction, where functional group gets a lower number. The third step is addition of substituents at appropriate atoms.
Answer to Problem 22.5P
The structure corresponding to the given name is,
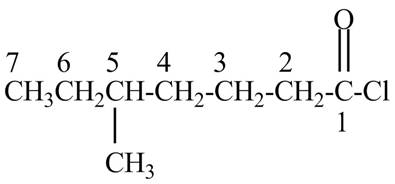
Explanation of Solution
The given name indicates that compound contains acid chloride (name ends with –yl chloride) as the functional group and heptane as the longest carbon chain with one methyl substitution at
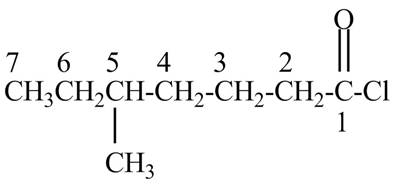
Figure 1
The structure corresponding to the given name is drawn in Figure 1.
(b)
Interpretation: The structure corresponding to the given name is to be drawn.
Concept introduction: One should follow the given steps to draw the structure of a compound from its name. The first step is identification of parent name and functional group found at the end of the name. The second step is numbering of carbon skeleton in the direction, where functional group gets a lower number. The third step is addition of substituents at appropriate atoms.
Answer to Problem 22.5P
The structure corresponding to the given name is,
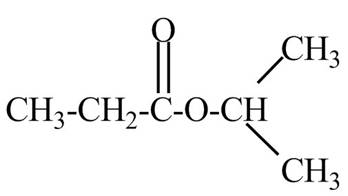
Explanation of Solution
The given name indicates that compound contains ester (name ends with –ate) as the functional group and propane as the longest carbon chain with one isopropyl substitution as
Thus, the structure of isopropyl propanoate is drawn as follows.
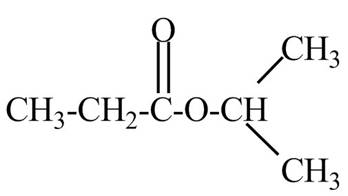
Figure 2
The structure corresponding to the given name is drawn in Figure 2.
(c)
Interpretation: The structure corresponding to the given name is to be drawn.
Concept introduction: One should follow the given steps to draw the structure of a compound from its name. The first step is identification of parent name and functional group found at the end of the name. The second step is numbering of carbon skeleton in the direction, where functional group gets a lower number. The third step is addition of substituents at appropriate atoms.
Answer to Problem 22.5P
The structure corresponding to the given name is,
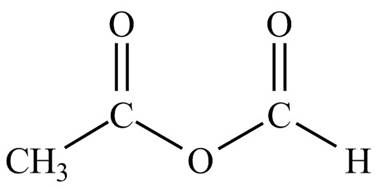
Explanation of Solution
The given name indicates that compound contains anhydride (name ends with -anhydride) as the functional group, which is derived from acetic acid and formic acid. Thus, it is a mixed anhydride. The structures of mixed anhydrides are drawn by joining the one ends of
Hence, the structure of acetic formic anhydride is drawn as follows.
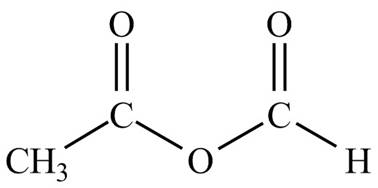
Figure 3
The structure corresponding to the given name is drawn in Figure 3.
(d)
Interpretation: The structure corresponding to the given name is to be drawn.
Concept introduction: One should follow the given steps to draw the structure of a compound from its name. The first step is identification of parent name and functional group found at the end of the name. The second step is numbering of carbon skeleton in the direction, where functional group gets a lower number. The third step is addition of substituents at appropriate atoms.
Answer to Problem 22.5P
The structure corresponding to the given name is,
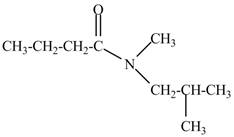
Explanation of Solution
The given name indicates that compound contains amide (name ends with -amide) as the functional group and butane as the longest carbon chain with one methyl and one isobutyl substitutions at
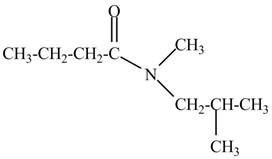
Figure 4
The structure corresponding to the given name is drawn in Figure 4.
(e)
Interpretation: The structure corresponding to the given name is to be drawn.
Concept introduction: One should follow the given steps to draw the structure of a compound from its name. The first step is identification of parent name and functional group found at the end of the name. The second step is numbering of carbon skeleton in the direction, where functional group gets a lower number. The third step is addition of substituents at appropriate atoms.
Answer to Problem 22.5P
The structure corresponding to the given name is,
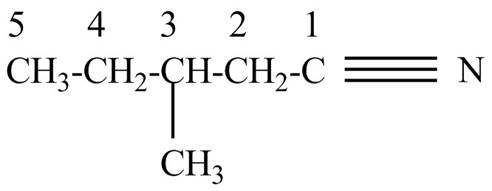
Explanation of Solution
The given name indicates that compound contains cyano (name ends with –nitrile) as the functional group and pentane as the longest carbon chain with one methyl substitution at

Figure 5
The structure corresponding to the given name is drawn in Figure 5.
(f)
Interpretation: The structure corresponding to the given name is to be drawn.
Concept introduction: One should follow the given steps to draw the structure of a compound from its name. The first step is identification of parent name and functional group found at the end of the name. The second step is numbering of carbon skeleton in the direction, where functional group gets a lower number. The third step is addition of substituents at appropriate atoms.
Answer to Problem 22.5P
The structure corresponding to the given name is,
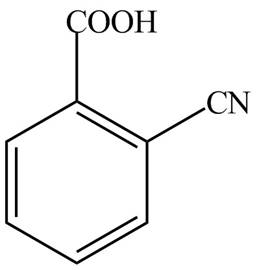
Explanation of Solution
The given name indicates that compound contains carboxylic acid (name ends with -oic acid) as the functional group, which is attached to the benzene ring with one cyano group at ortho position. Therefore, the structure of o-cyanobenzoic acid is drawn as follows.

Figure 6
The structure corresponding to the given name is drawn in Figure 6.
(g)
Interpretation: The structure corresponding to the given name is to be drawn.
Concept introduction: One should follow the given steps to draw the structure of a compound from its name. The first step is identification of parent name and functional group found at the end of the name. The second step is numbering of carbon skeleton in the direction, where functional group gets a lower number. The third step is addition of substituents at appropriate atoms.
Answer to Problem 22.5P
The structure corresponding to the given name is,
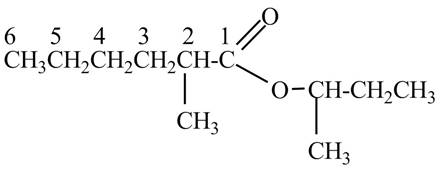
Explanation of Solution
The given name indicates that compound contains ester (name ends with –ate) as the functional group and hexane as the longest carbon chain with one methyl substitution at

Figure 7
The structure corresponding to the given name is drawn in Figure 7.
(h)
Interpretation: The structure corresponding to the given name is to be drawn.
Concept introduction: One should follow the given steps to draw the structure of a compound from its name. The first step is identification of parent name and functional group found at the end of the name. The second step is numbering of carbon skeleton in the direction, where functional group gets a lower number. The third step is addition of substituents at appropriate atoms.
Answer to Problem 22.5P
The structure corresponding to the given name is,
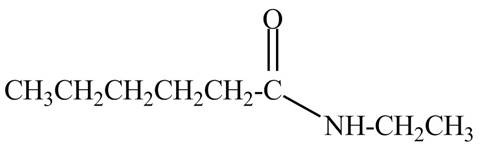
Explanation of Solution
The given name indicates that compound contains amide (name ends with -amide) as the functional group and hexane is the longest carbon chain with one ethyl substitution to
Therefore, the structure of N-ethylhexanamide is drawn as follows.

Figure 8
The structure corresponding to the given name is drawn in Figure 8.
Want to see more full solutions like this?
Chapter 22 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
- Please help me answer this question. I don't understand how or even if this can happen in a single transformation. Please provide a detailed explanation and a drawing showing how it can happen in a single transformation. Add the necessary reagents and reaction conditions above and below the arrow in this organic reaction. If the products can't be made from the reactant with a single transformation, check the box under the drawing area instead.arrow_forward2) Draw the correct chemical structure (using line-angle drawings / "line structures") from their given IUPAC name: a. (E)-1-chloro-3,4,5-trimethylhex-2-ene b. (Z)-4,5,7-trimethyloct-4-en-2-ol C. (2E,6Z)-4-methylocta-2,6-dienearrow_forwardපිපිම Draw curved arrows to represent the flow of electrons in the reaction on the left Label the reactants on the left as either "Acid" or "Base" (iii) Decide which direction the equilibrium arrows will point in each reaction, based on the given pk, values (a) + H-O H 3-H + (c) H" H + H****H 000 44-00 NH₂ (e) i Дон OH Ө NHarrow_forward
- 3) Label the configuration in each of the following alkenes as E, Z, or N/A (for non-stereogenic centers). 00 E 000 N/A E Br N/A N/A (g) E N/A OH E (b) Oz N/A Br (d) 00 E Z N/A E (f) Oz N/A E (h) Z N/Aarrow_forward6) Fill in the missing Acid, pKa value, or conjugate base in the table below: Acid HCI Approximate pK, -7 Conjugate Base H-C: Hydronium (H₂O') -1.75 H-O-H Carboxylic Acids (RCOOH) Ammonium (NH4) 9.24 Water (H₂O) H-O-H Alcohols (ROH) RO-H Alkynes R--H Amines 25 25 38 HOarrow_forward5) Rank the following sets of compounds in order of decreasing acidity (most acidic to least acidic), and choose the justification(s) for each ranking. (a) OH V SH я вон CH most acidic (lowst pKa) least acidic (highest pKa) Effect(s) Effect(s) Effect(s) inductive effect O inductive effect O inductive effect electronegativity electronegativity O electronegativity resonance polarizability resonance polarizability O resonance O polarizability hybridization Ohybridization O hybridization оarrow_forward
- How negatively charged organic bases are formed.arrow_forwardNonearrow_forward1) For the following molecules: (i) Label the indicated alkenes as either cis (Z), trans (E), or N/A (for non-stereogenic centers) by bubbling in the appropriate label on the molecule. (ii) Complete the IUPAC name located below the structure (HINT: Put the letter of the configuration in parentheses at the beginning of the name!) E z N/A ()-3,4,6-trimethylhept-2-ene E Oz O N/A ()-3-ethyl-1-fluoro-4-methylhex-3-ene E -+- N/A Me )-2,3-dimethylpent-2-ene (d) (b) E O N/A Br ()-5-bromo-1-chloro-3-ethyloct-4-ene ОЕ Z N/A Et (___)-3-ethyl-4-methylhex-3-ene E (f) Oz N/A z N/A HO (4.7)-4-(2-hydroxyethyl)-7-methylnona-4,7-dien-2-onearrow_forward
- O 9:21AM Tue Mar 4 ## 64% Problem 51 of 15 Submit Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. H :0: CI. AI :CI: :CI: Cl AI Select to Add Arrows Select to Add Arrows O: Cl :CI: :0: H CI: CI CO Select to Add Arrows Select to Add Arrows :O: CI :0: Cl. 10: AIarrow_forward(i) Draw in the missing lone pair(s) of electrons of the reactants on the left (ii) Draw (curved) arrows to show the flow of electrons in the acid/base reaction on the left (iii) Draw the products of the acid/base on the right (iv) Select the correct label for each product as either "conjugate acid" or "conjugate base" (a) JOH OH NH₂ acid base (b) De "H conjugate acid conjugate acid conjugate base conjugate base acid base conjugate acid conjugate base conjugate acid conjugate base acid basearrow_forwardCould someone answer this NMR and explain please Comment on the general features of the 1H-NMR spectrum of isoamyl ester provided below.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





