
Concept explainers
Identify the structures of each compound from the given data.
a. Molecular formula
IR absorption:
(quartet,
b. Molecular formula
IR absorption:
(triplet,
c. Molecular formula
IR absorption:
d. Molecular formula
IR absorption:
(triplet,
e. Molecular formula
IR absorption:
(a)
Interpretation: The structure of compound that has molecular formula
Concept introduction: The method of spectroscopy is used to identify the structure of the molecule. It is based on the interactions between matter and electromagnetic radiations. Proton NMR spectroscopy identifies the number of hydrogen atoms present in a molecule and the nature of the functional group. The value of chemical peaks depends upon the chemical environment around the hydrogen atom.
Answer to Problem 22.75P
The structure of given compound is,
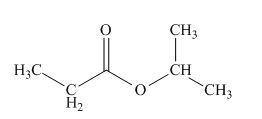
Explanation of Solution
The IR absorption value of the given compound is
A septet of one hydrogen atoms at
A triplet peak of three hydrogen atoms and a quartet of two carbon atoms shows that their carbon atoms are bonded to each other and the splitting peak takes place according to
Therefore, the structure of compound is,

Figure 1
The structure of given compound is shown in Figure 1.
(b)
Interpretation: The structure of compound that has molecular formula
Concept introduction: The method of spectroscopy is used to identify the structure of the molecule. It is based on the interactions between matter and electromagnetic radiations. Proton NMR spectroscopy identifies the number of hydrogen atoms present in a molecule and the nature of the functional group. The value of chemical peaks depends upon the chemical environment around the hydrogen atom.
Answer to Problem 22.75P
The structure of given compound is,
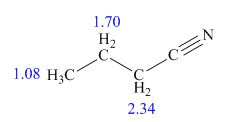
Explanation of Solution
The IR absorption value of the given compound is
In the given compound, one out of four carbon atoms is
Therefore, the structure of compound is,

Figure 2
The structure of given compound is shown in Figure 2.
(c)
Interpretation: The structure of compound that has molecular formula
Concept introduction: The method of spectroscopy is used to identify the structure of the molecule. It is based on the interactions between matter and electromagnetic radiations. Proton NMR spectroscopy identifies the number of hydrogen atoms present in a molecule and the nature of the functional group. The value of chemical peaks depends upon the chemical environment around the hydrogen atom.
Answer to Problem 22.75P
The structure of given compound is,
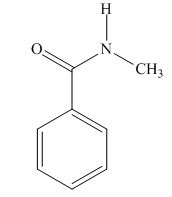
Explanation of Solution
The IR absorption value of the given compound are
Multiple
A singlet NMR peak of one hydrogen atom is observed due to presence of
Therefore, the structure of compound is,

Figure 3
The structure of given compound is shown in Figure 3.
(d)
Interpretation: The structure of compound that has molecular formula
Concept introduction: The method of spectroscopy is used to identify the structure of the molecule. It is based on the interactions between matter and electromagnetic radiations. Proton NMR spectroscopy identifies the number of hydrogen atoms present in a molecule and the nature of the functional group. The value of chemical peaks depends upon the chemical environment around the hydrogen atom.
Answer to Problem 22.75P
The structure of given compound is,
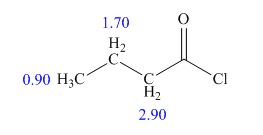
Explanation of Solution
The IR absorption value of the given compound is
In the given compound, one out of four carbon atoms is
Therefore, the structure of compound is,

Figure 4
The structure of given compound is shown in Figure 4.
(e)
Interpretation: The structure of compound that has molecular formula
Concept introduction: The method of spectroscopy is used to identify the structure of the molecule. It is based on the interactions between matter and electromagnetic radiations. Proton NMR spectroscopy identifies the number of hydrogen atoms present in a molecule and the nature of the functional group. The value of chemical peaks depends upon the chemical environment around the hydrogen atom.
Answer to Problem 22.75P
The structure of given compound is,
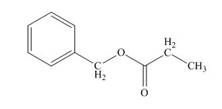
Explanation of Solution
The IR absorption value of the given compound is
Multiple
Therefore, the structure of compound is,
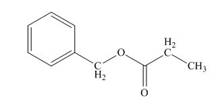
Figure 5
The structure of given compound is shown in Figure 5.
Want to see more full solutions like this?
Chapter 22 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
- Consider this reaction (molecular weights are under each compound): HC=CH + 2 HCI --> C2H4Cl 2 MW = 26 36.5 99 If 4.4 g of HC=CH are reacted with 110 mL of a 2.3 M HCI solution, and 6.0 g of product are actually produced, what is the percent yield?arrow_forwardWhat is the name of the major product of this reaction? OH CH3 H₂SO4, heat 1-methylcyclohexene O2-methyl-1-cyclohexene O 3-mthylcyclohexene 1-methyl-2-cyclohexenearrow_forwardWe added a brown solution of Br2 to one of our products, and the brown color disappeared. This indicated that our product wasarrow_forward
- Rank the following according to reactivity toward nitration: a) benzene b) bromobenzene c) nitrobenzene d) phenol Od) greatest, c) least Od) greatest, b) least Od) greatest, a) least a) greatest, b) least a) greatest, c) least Oa) greatest, d) least Ob) greatest, a) least O b) greatest, c) least Ob) greatest, d) least O c) greatest, a) least O c) greatest, b) least O c) greatest, d) leastarrow_forwardO-Nitrophenol was distilled over with the steam in our experiment while the other isomer did not. This is due to: O intramolecular hydrogen bonding in the ortho isomer O intermolecular hydrogen bonding in the the ortho isomer O the ortho isomer has a lower density O the ortho isomer has a lower molecular weightarrow_forwardK 44% Problem 68 of 15 Submit Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. :6: :: :CI: CI CI: :0:0 Select to Add Arrows Select to Add Arrows H H Cl CI: CI CI: Select to Add Arrows Select to Add Arrows H :CI: Alarrow_forward
- I I H :0: Submit Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. 0:0 :0: CI ΑΙ :CI: :CI: :0: CI Select to Add Arrows Select to Add Arrows cl. :0: Cl © ハ CI:: CI H CO Select to Add Arrows Select to Add Arrows 10: AI ::arrow_forwardOrder the following compounds from slowest to fastest in a nucleophilic acyl substitution reaction. ii 요 OB D A E C OCE Darrow_forwardI need the most help figuring out how to find [I^-] mol/ L, [S2O8^2-] mol/L. 1st and 2nd Blank columns.arrow_forwardCan someone help me whats the issue?arrow_forwarda. The change in the Gibbs energy of a certain constant pressure process is found to fit the expression: AG-85.1 J mol −1 +36.5 J mol ¹K-1 × T A. Calculate the value of AS for the process. B. Next, use the Gibbs-Helmholtz equation: (a(AG/T)) ΔΗ - T2 to calculate the value of AH for the process.arrow_forwardNonearrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
