
(a)
Interpretation: The acceptable names for the given compounds are to be predicted.
Concept introduction:
• The hydrocarbon is named after the longest carbon chain.
• The parent hydrocarbon containing ester group is named with suffix “ate”.
• The alkyl group bonded to oxygen through single bond forms the first part of the ester and alkyl group bonded to carbonyl group forms the second part.
Answer to Problem 22.38P
The acceptable name of the compound A is
Explanation of Solution
The given compound A is,
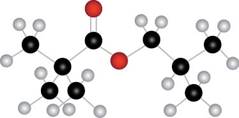
Figure 1
The red coloured ball has two bonds. So, this is the oxygen atom. The black coloured atoms have four single bonds. So, these are the carbon atoms. The grey coloured balls have one bond. So, these are the hydrogen atoms. The molecular structure of the compound is,
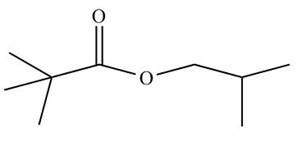
Figure 2
This compound contains ester group. The alkyl group attached to carbonyl group has longest chain of three carbon atoms with two methyl groups substituted at the second carbon atom. The alkyl group bonded to oxygen through single bond has longest chain of three carbon atoms with one methyl group substituted at the second carbon atom. The acceptable name of the compound is
The given compound B is,
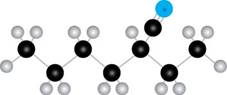
Figure 3
The blue coloured ball has three bonds. So, this is the nitrogen atom. The black coloured atoms have four bonds. So, these are the carbon atoms. The grey coloured balls have one bond. So, these are the hydrogen atoms. The molecular structure of the compound is,
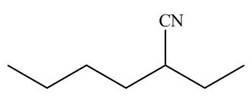
Figure 4
The given compound B contains longest chain of six carbon atoms with ethyl group substituted at the second carbon atom. The acceptable name of the compound is
The acceptable name of the compound A is
(b)
Interpretation: The products formed on treatment of A or B with given reagents: [1]
Concept introduction: The metal hydride reagents are good reducing agents such as
When the esters and nitriles are hydrolyzed to carboxylic acids in acidic medium and in basic medium carboxylate ions are formed.
Grignard reagents are organometallic compounds which are prepared using alkyl halides in the presence of magnesium metal in dry ether. These reagents act as strong nucleophiles and bases.
Answer to Problem 22.38P
The reaction of compound A with given reagents are shown below.
[1]
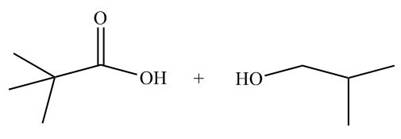
Figure 5
[2]
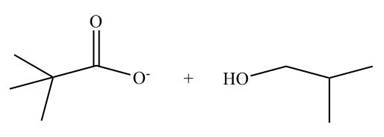
Figure 6
[3]
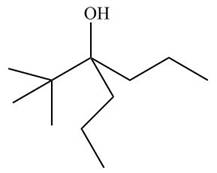
Figure 7
[4]
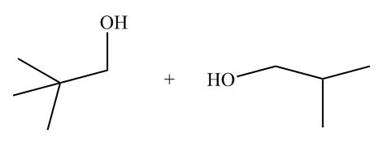
Figure 8
The reaction of compound B with given reagents are shown below.
[1]
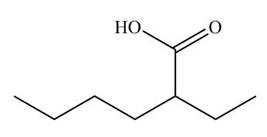
Figure 9
[2]
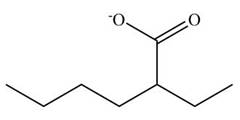
Figure 10
[3]
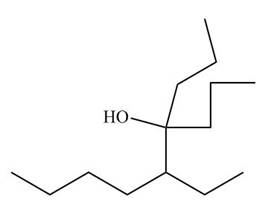
Figure 11
[4]
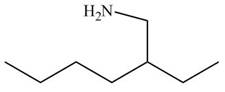
Figure 12
Explanation of Solution
The reaction of compound A with given reagents are shown below.
[1]
The ester group undergoes acidic hydrolysis to form alcohol and
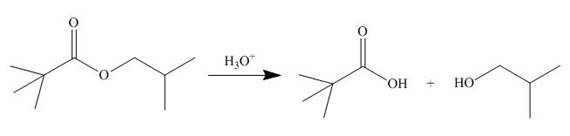
Figure 13
The products formed are
[2]
The ester group undergoes basic hydrolysis to form alcohol and carboxylic acid as shown below.
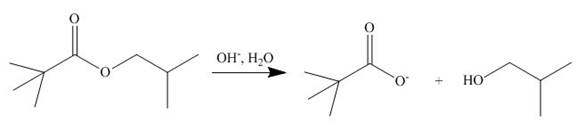
Figure 14
The products formed are
[3]
The given ester compound reacts with excess Grignard reagent followed by hydrolysis to form
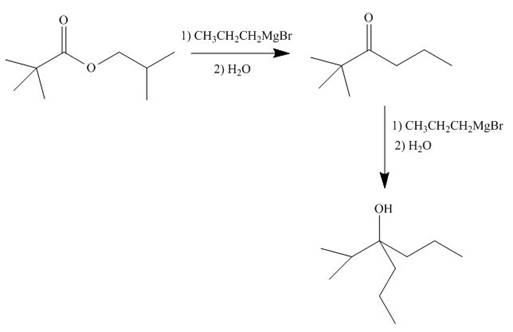
Figure 15
The product formed is form
[4]
In presence of lithium aluminium hydride, the ester group is reduced to two alcohols. The corresponding reaction is shown below.
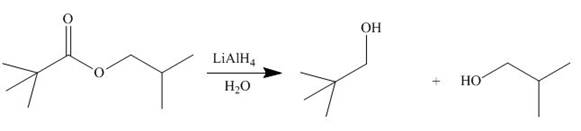
Figure 16
The products formed are
The reaction of compound B with given reagents are shown below.
[1]
The cyano group undergoes acidic hydrolysis to form carboxylic acid as shown below.
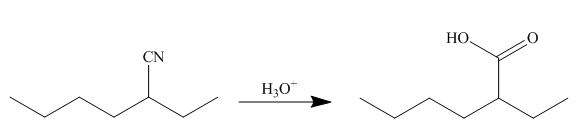
Figure 17
The products formed is
[2]
The cyano group undergoes basic hydrolysis to form carboxylate ion as shown below.
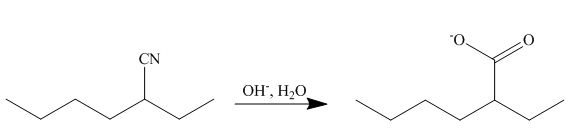
Figure 18
The product formed is
[3]
The nitrile compound reacts with Grignard reagent to form alcohol. The corresponding reaction is shown below.
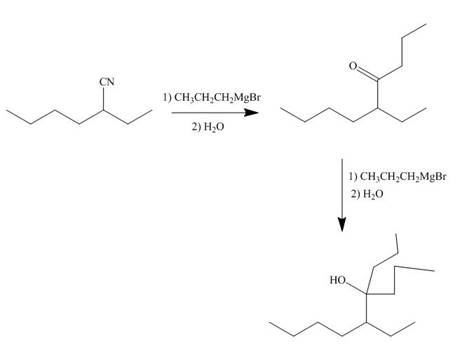
Figure 19
[4]
In the presence of lithium aluminium hydride, the ester group is reduced to two alcohols. The corresponding reaction is shown below.
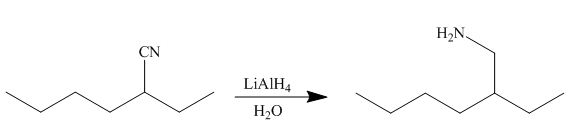
Figure 20
The product formed is
The products formed on treatment of A or B with given reagents: [1]
Want to see more full solutions like this?
Chapter 22 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
- The number of imaginary replicas of a system of N particlesA) can never become infiniteB) can become infiniteC) cannot be greater than Avogadro's numberD) is always greater than Avogadro's number.arrow_forwardElectronic contribution to the heat capacity at constant volume A) is always zero B) is zero, except for excited levels whose energy is comparable to KT C) equals 3/2 Nk D) equals Nk exp(BE)arrow_forwardPlease correct answer and don't used hand raitingarrow_forward
- Calculate the packing factor of CaTiO3. It has a perovskite structure. Data: ionic radii Co²+ = 0.106 nm, Ti4+ = 0.064 nm, O² = 0.132 nm; lattice constant is a = 2(rTi4+ + ro2-). Ca2+ 02- T14+ Consider the ions as rigid spheres. 1. 0.581 or 58.1% 2. -0.581 or -58.1 % 3. 0.254 or 25.4%arrow_forwardGeneral formula etherarrow_forwardPlease provide the retrosynthetic analysis and forward synthesis of the molecule on the left from the starting material on the right. Please include hand-drawn structures! will upvote! Please correct answer and don't used hand raitingarrow_forward
- Please provide the retrosynthetic analysis and forward synthesis of the molecule on the left from the starting material on the right. Please include hand-drawn structures! will upvote!arrow_forward(please correct answer and don't used hand raiting) Please provide the retrosynthetic analysis and forward synthesis of the molecule on the left from the starting material on the right. Please include hand-drawn structures! will upvote!arrow_forwardCaTiO3 has a perovskite structure. Calculate the packing factor.Data: ionic radii Co+2 = 0.106 nm, Ti+4 = 0.064 nm, O-2 = 0.132 nm; lattice constant is a = 2(rTi4+ + rO-2).(a) 0.581(b) -0.581(c) 0.254(d) -0.254arrow_forward
- In the initial linear section of the stress-strain curve of a metal or alloy. Explain from the point of view of atomic structure?(a) No, the atomic level properties of the material can never be related to the linear section.(b) The elastic zone is influenced by the strength of the bonds between atoms.(c) The stronger the bond, the less rigid and the lower the Young's Modulus of the material tested.(d) The stronger the bond, the less stress is necessary to apply to the material to deform it elastically.arrow_forwardThe degree of polymerization of polytetrafluoroethylene (Teflon) is 7500 (mers/mol). If all polymer chains have equal length, state the molecular weight of the polymer and the total number of chains in 1000 g of the polymer(a) 50 000 g/mol; 0.03·1020 chains(b) 100 000 g/mol; 1.03·1020 chains(c) 750 000 g/mol; 8.03·1020 chainsarrow_forwardIn natural rubber or polyisoprene, the trans isomer leads to a higher degree of crystallinity and density than the cis isomer of the same polymer, because(a) it is more symmetrical and regular.(b) it is less symmetrical.(c) it is irregular.arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

