
Concept explainers
Give the structure corresponding to each name.
a. cyclohexyl propanoate
b. cyclohexanecarboxamide
c.
d. Vinyl acetate
e. Benzoic propanoic anhydride
f.
g. octyl butanoate
h. N, N-dibenzylformamide
(a)
Interpretation: The structure corresponding to the given name is to be drawn.
Concept introduction: One should follow the given steps to draw the structure of a compound from its name. The first step is identification of parent name and functional group found at the end of the name. The second step is numbering of carbon skeleton in the direction, where functional group gets a lower number. The third step is addition of substituents at appropriate atoms.
Answer to Problem 22.41P
The structure corresponding to the given name is
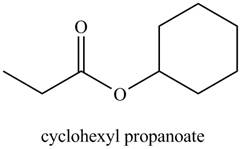
Explanation of Solution
The given name indicates that compound contains ester (name ends with –ate) as the functional group, and propane as the longest carbon chain with one cyclohexyl substitution as
Thus, the structure corresponding to the given name is,
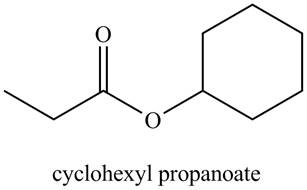
Figure 1
The structure corresponding to the given name is drawn in Figure 1.
(b)
Interpretation: The structure corresponding to the given name is to be drawn.
Concept introduction: One should follow the given steps to draw the structure of a compound from its name. The first step is identification of parent name and functional group found at the end of the name. The second step is numbering of carbon skeleton in the direction, where functional group gets a lower number. The third step is addition of substituents at appropriate atoms.
Answer to Problem 22.41P
The structure corresponding to the given name is,
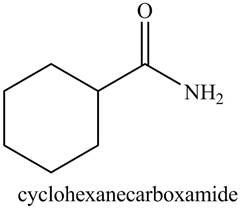
Explanation of Solution
The given name indicates that compound contains amide (name ends with -amide) as the functional group. This amide group is derived from cyclohexylcarboxylic acid, and bonded to cyclohexane ring.
Thus, the structure corresponding to given name is,
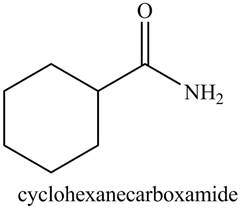
Figure 2
The structure corresponding to the given name is drawn in Figure 2.
(c)
Interpretation: The structure corresponding to the given name is to be drawn.
Concept introduction: One should follow the given steps to draw the structure of a compound from its name. The first step is identification of parent name and functional group found at the end of the name. The second step is numbering of carbon skeleton in the direction, where functional group gets a lower number. The third step is addition of substituents at appropriate atoms.
Answer to Problem 22.41P
The structure corresponding to the given name is,
Explanation of Solution
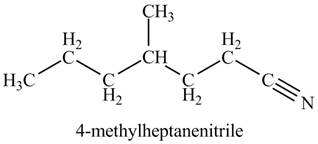
The given name indicates that compound contains cyano (name ends with -nitrile) as the functional group, and heptane as the longest carbon chain with one methyl substitution at
Thus, the structure corresponding to given name is,
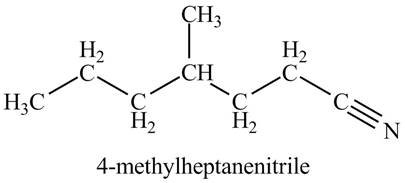
Figure 3
The structure corresponding to the given name is drawn in Figure 3.
(d)
Interpretation: The structure corresponding to the given name is to be drawn.
Concept introduction: One should follow the given steps to draw the structure of a compound from its name. The first step is identification of parent name and functional group found at the end of the name. The second step is numbering of carbon skeleton in the direction, where functional group gets a lower number. The third step is addition of substituents at appropriate atoms.
Answer to Problem 22.41P
The structure corresponding to the given name is,
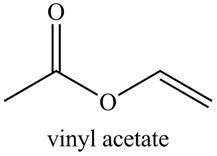
Explanation of Solution
The given name indicates that compound contains ester (name ends with -ate) as the functional group, and an acetate group is bonded to a vinyl group
Thus, the structure corresponding to the given name is,
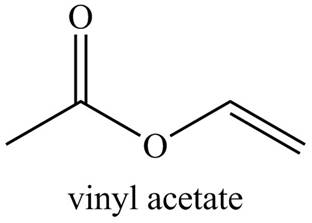
Figure 4
The structure corresponding to the given name is drawn in Figure 4.
Interpretation: The structure corresponding to the given name is to be drawn.
(e)
Concept introduction: One should follow the given steps to draw the structure of a compound from its name. The first step is identification of parent name and functional group found at the end of the name. The second step is numbering of carbon skeleton in the direction, where functional group gets a lower number. The third step is addition of substituents at appropriate atoms.
Answer to Problem 22.41P
The structure corresponding to the given name is,
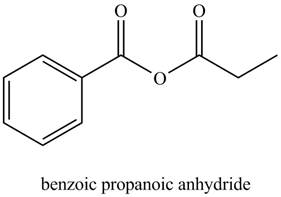
Explanation of Solution
The given name indicates that compound contains anhydride (name ends with -anhydride) as the functional group. This anhydride compound is derived from benzoic acid and propanoic acid.
Thus, the structure corresponding to the given name is,

Figure 5
The structure corresponding to the given name is drawn in Figure 5.
Interpretation: The structure corresponding to the given name is to be drawn.
(f)
Concept introduction: One should follow the given steps to draw the structure of a compound from its name. The first step is identification of parent name and functional group found at the end of the name. The second step is numbering of carbon skeleton in the direction, where functional group gets a lower number. The third step is addition of substituents at appropriate atoms.
Answer to Problem 22.41P
The structure corresponding to the given name is,
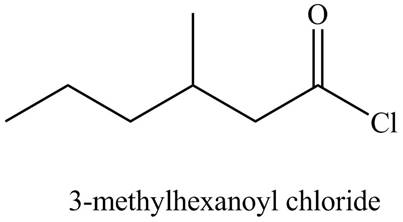
Explanation of Solution
The given name indicates that compound contains acid chloride (name ends with -yl chloride) as the functional group, and hexane as the longest carbon chain with one methyl substitution at
Thus, the structure corresponding to the given name is,
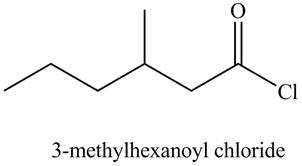
Figure 6
The structure corresponding to the given name is drawn in Figure 6.
Interpretation: The structure corresponding to the given name is to be drawn.
(g)
Concept introduction: One should follow the given steps to draw the structure of a compound from its name. The first step is identification of parent name and functional group found at the end of the name. The second step is numbering of carbon skeleton in the direction, where functional group gets a lower number. The third step is addition of substituents at appropriate atoms.
Answer to Problem 22.41P
The structure corresponding to the given name is,
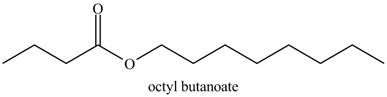
Explanation of Solution
The given name indicates that compound contains ester (name ends with –ate) as the functional group, and butane as the longest carbon chain with one octyl substitution as
Thus, the structure corresponding to the given name is,

Figure 7
The structure corresponding to the given name is drawn in Figure 7.
(h)
Interpretation: The structure corresponding to the given name is to be drawn.
Concept introduction: One should follow the given steps to draw the structure of a compound from its name. The first step is identification of parent name and functional group found at the end of the name. The second step is numbering of carbon skeleton in the direction, where functional group gets a lower number. The third step is addition of substituents at appropriate atoms.
Answer to Problem 22.41P
The structure corresponding to the given name is,
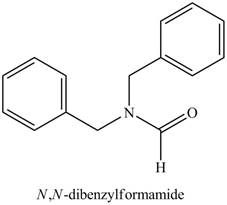
Explanation of Solution
The given name indicates that compound contains amide (name ends with -amide) as the functional group. This amide group is derived from formic acid, and bonded to two benzyl groups.
Thus, the structure corresponding to the given name is,
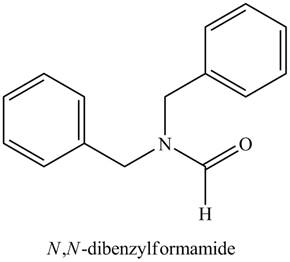
Figure 8
The structure corresponding to the given name is drawn in Figure 8.
Want to see more full solutions like this?
Chapter 22 Solutions
Package: Loose Leaf for Organic Chemistry with Biological Topics with Connect Access Card
- 90. Draw the stereoisomers obtained from each of the following reactions: a. H₂ b. H₂ C. H₂ Pd/C Pd/C Pd/Carrow_forward36. The emission spectrum below for a one-electron (hydrogen-like) species in the gas phase shows all the lines, before they merge together, resulting from transitions to the first excited state from higher energy states. Line A has a wavelength of 434 nm. BA Increasing wavelength, λ (a) What are the upper and lower principal quantum numbers corresponding to the lines labeled A and B? (b) Identify the one-electron species that exhibits the spectrum.arrow_forwardf) The unusual molecule [2.2.2] propellane is pictured. 1) Given the bond length and bond angles in the image, what hybridization scheme best describes the carbons marked by the askerisks? 2) What types of orbitals are used in the bond between the two carbons marked by the askerisks? 3) How does this bond compare to an ordinary carbon-carbon bond (which is usually 1.54 Å long)? H₂C H₂C CH2 1.60Å ハ C. * CH₂ H₂C * C H₂ 120°arrow_forward
- Question Resonance Forms a) Draw all resonance forms of the molecules. Include curved arrow notation. Label major resonance contributor Resonance Forms a) Draw all resonance forms of the molecules. Include curved arrow notation. Label major resonance contributorarrow_forwardCan you show me or determine the longest carbon chain, which is octane? Potentially highlight it in different sections to show me, plz, or individually?arrow_forwardPLEASE ANSWER ALL PARTS!!arrow_forward
- d) Determine the formal charge on the nitrogen atom in each of the structures. NH3 NH2 N C бобкат : N N H H Н H2N-OH A B C D E F Garrow_forwardLewis Structure, Hybridization & Molecular Geometry a) Draw the Lewis Structure of the molecules; Label the hybridization of each carbon atom; Predict the approximate molecular geometry around each carbon atom. CH3CHO CH3CN b) Draw the Lewis Structure of Nitromethane; Predict the approximate molecular geometry around the nitrogen atom. CH3NO2 c) Draw the Lewis Structure; Label the hybridization of the boron atom; Predict the approximate molecular geometry. BF3 BF4arrow_forwarda. The structure of the bicarbonate (hydrogen carbonate) ion, HCO3-, HCO3 " is best described as a hybrid of several contributing resonance forms, two of which are shown here. HO :0: HO + :Ö: Bicarbonate is crucial for the control of body pH (for example, blood pH 7.4). A more self-indulgent use is in baking soda, where it serves as a source of CO2 CO2 gas, which gives bread and pastry their fluffy constituency. (i) Draw at least one additional resonance form. = (ii) Using curved "electron-pushing" arrows, show how these Lewis structures may be interconverted by movement of electron pairs. (iii) Determine which form or forms will be the major contributor(s) to the real structure of bicarbonate, explaining your answer on the basis of the criteria in Section 1-5.arrow_forward
- Calibri 11 + BIL NAME: Jaylena M A student is investigating the ctect of volume on pressure during a lab activity. The student uses the following volumes (mL). 12, 13, 14, 16, 17, 18, 19, 20, 21, 22, 23, 24, 26, 28, 30, 33, 34, 35, 38, 40, 42, 44. 46, and 50. As the volume changed they measured the following pressures (atm) 11.0, 10.5, 10.0, 9.2. 8.5, 78, 75, 7.0, 6.8, 6.5, 6.0, 5.9, 5.5, 5.0, 4.8, 4.5, 4.2, 3.9, 3.8, 3.5, 3.3, 3.2, 3.0, 2.9. What is the independent variable? Volume Imla What is the dependent variable? Pressure Jatm Use the data and make a PROPER data table. Volume 1mL) Pressure latm 110arrow_forwardDraw all resonance forms of the molecules. Include curved arrow notation. Label major resonance contributor.arrow_forward: Resonance Forms a) Draw all resonance forms of the molecules. Include curved arrow notation. Label major resonance contributor. SO₂ NO3arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





