
General, Organic, and Biological Chemistry - 4th edition
4th Edition
ISBN: 9781259883989
Author: by Janice Smith
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 22, Problem 20P
(a) Give the name of each compound shown as a ball-and-stick model. (b) Classify the compound as a base, nucleoside, or 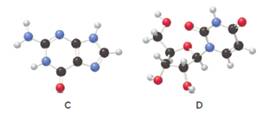
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
3.
An unknown element, X, combines with chlorine to give a substance with the formula
XC14. A chlorine analysis of the substance indicates that it contains 83.47% chlorine by mass.
What element is X and what is the formula of this compound?
(Hint: to identify an element or compound, identify its molar mass. Remember that Molar Mass
= (grams A)/(moles A). Solve for each individually and then divide them to find molar mass.)
1.
When hydrogen sulfide (H2S, MM = 34.08 g/mol) gas is bubbled into a solution of
sodium hydroxide (NaOH, 40.00 g/mol), sodium sulfide (Na2S, 78.04 g/mol) and water (18.02
g/mol) are produced according to the balanced chemical equation shown below?
H2S 2 NaOH --> Na2S 2 H₂O
(a) Assuming the reaction goes to completion, how many grams of sodium sulfide are formed if
2.50g of hydrogen sulfide is bubbled into a solution containing 1.85g of NaOH? (20 pts)
(b) Which reactant and how much of it remains after the reaction has been completed? (15 pts)
(c) If only 0.400g of sodium sulfide was recovered, what is the percent yield of this reaction (5
pts)
The organic compound MTBE (methyltertiarybutylether) is used as a fuel additive that
allows gasoline to burn more cleanly thus leading to a reduction in pollution. Recently,
however, MTBE has been found in the drinking water of a number of communities. As a
result several states are phasing out the use of MTBE as a fuel additive. A combustion
experiment using 10.00 g of MTBE was found to produce 24.97g of CO2 and 12.26 g of
H2O.
(a) What is the empirical formula of MTBE assuming it contains C, H, and O only?
(b) The molar mass of MTBE was experimentally determined to be 88.1 g/mol. Using this
information what is the molecular formula of MTBE
Chapter 22 Solutions
General, Organic, and Biological Chemistry - 4th edition
Ch. 22.1 - Prob. 22.1PPCh. 22.1 - Prob. 22.1PCh. 22.1 - -Fluorouracil is an anticancer drug that...Ch. 22.1 - Prob. 22.3PCh. 22.1 - Prob. 22.4PCh. 22.1 - Label each statement about the compound...Ch. 22.1 - Draw the structure of each nucleotide: (a) UMP;...Ch. 22.1 - Give the name that corresponds to each...Ch. 22.2 - Draw the structure of a dinucleotide formed by...Ch. 22.2 - Draw the structure of each polynucleotide: (a) CU;...
Ch. 22.2 - Label each statement about the polynucleotide...Ch. 22.3 - Write the complementary strand for each of the...Ch. 22.4 - What is the sequence of a newly synthesized DNA...Ch. 22.6 - For each DNA segment: [1] What is the sequence of...Ch. 22.6 - Prob. 22.9PCh. 22.7 - What amino acid is coded for by each codon? GCC...Ch. 22.7 - What codons code for each amino acid? a. glycine...Ch. 22.7 - Drive the amino acid sequence that is coded for by...Ch. 22.7 - Write a possible mRNA sequence that codes for each...Ch. 22.7 - Considering the given sequence of nucleotides in...Ch. 22.8 - Prob. 22.14PCh. 22.8 - Prob. 22.8PPCh. 22.8 - Prob. 22.9PPCh. 22.9 - Prob. 22.10PPCh. 22.9 - Prob. 22.15PCh. 22.10 - Prob. 22.16PCh. 22 - Label each statement as pertaining to DNA, RNA, or...Ch. 22 - Label each statement as pertaining to DNA, RNA, or...Ch. 22 - Prob. 19PCh. 22 - (a) Give the name of each compound shown as a...Ch. 22 - Prob. 21PCh. 22 - Prob. 22PCh. 22 - Prob. 23PCh. 22 - Prob. 24PCh. 22 - Prob. 25PCh. 22 - Draw the structure of each of the following: a...Ch. 22 - Prob. 27PCh. 22 - Prob. 28PCh. 22 - Prob. 29PCh. 22 - Prob. 30PCh. 22 - Prob. 31PCh. 22 - Draw the structures of the two possible...Ch. 22 - Prob. 33PCh. 22 - Draw the structure of each dinucleotide and...Ch. 22 - Draw the deoxyribonucleotide TGA. Label the 5 and...Ch. 22 - Draw the ribonucleotide CGU. Label the 5 and 3...Ch. 22 - Prob. 37PCh. 22 - Describe in detail the DNA double helix with...Ch. 22 - Write the sequence of the complementary strand of...Ch. 22 - Prob. 40PCh. 22 - Prob. 41PCh. 22 - Prob. 42PCh. 22 - Prob. 43PCh. 22 - Prob. 44PCh. 22 - Prob. 45PCh. 22 - Figure 22.4 snows the hydrogen-bonding...Ch. 22 - Prob. 47PCh. 22 - Prob. 48PCh. 22 - Prob. 49PCh. 22 - Prob. 50PCh. 22 - What mRNA is transcribed from each DNA sequence in...Ch. 22 - Prob. 52PCh. 22 - For each DNA segment: [1] What is the sequence of...Ch. 22 - Prob. 54PCh. 22 - For each codon, give its anticodon and the amino...Ch. 22 - For each codon, give its anticodon and the amino...Ch. 22 - Fill in the missing information in the schematic...Ch. 22 - Fill in the missing information in the schematic...Ch. 22 - Derive the amino acid sequence that is coded for...Ch. 22 - Derive the amino acid sequence that is coded for...Ch. 22 - Write a possible mRNA sequence that codes for each...Ch. 22 - Prob. 62PCh. 22 - Prob. 63PCh. 22 - Prob. 64PCh. 22 - Prob. 65PCh. 22 - Prob. 66PCh. 22 - Consider the following mRNA sequence:...Ch. 22 - Consider the following mRNA sequence: 5-ACC UUA...Ch. 22 - Consider the following sequence of DNA: 3-TTA...Ch. 22 - Consider the following sequence of DNA: 3-ATA...Ch. 22 - Prob. 71PCh. 22 - Prob. 72PCh. 22 - Prob. 73PCh. 22 - Prob. 74PCh. 22 - Prob. 75PCh. 22 - Prob. 76PCh. 22 - Prob. 77PCh. 22 - Prob. 78PCh. 22 - Prob. 79PCh. 22 - Prob. 80PCh. 22 - Prob. 81PCh. 22 - Prob. 82PCh. 22 - Fill in the base, codon, anticodon, or amino acid...Ch. 22 - Fill in the base, codon, anticodon, or amino acid...Ch. 22 - Fill in the base, codon, anticodon, or amino acid...Ch. 22 - Prob. 86PCh. 22 - Prob. 87PCh. 22 - Prob. 88PCh. 22 - Prob. 89PCh. 22 - Prob. 90PCh. 22 - Prob. 91CPCh. 22 - Give a possible nucleotide sequence in the...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Part 4: Provide a detailed retrosynthetic analysis and a plausible forward synthesis the following molecule. храдо ofarrow_forward3A: Starting with benzocyclobutene, synthesize the naphthalene derivative below.arrow_forward7. The addition of HBr to 2,5-dimethyl-2,4-heptadiene gives the same product, A, at both low and high temperatures. Provide the structure of A and explain the kinetic and thermodynamic product are the same in this reaction. HBr -78°C or 60°C Aarrow_forward
- 3B: Convert the starting material into the chiral epoxytriol below. OH OH = OH OHarrow_forward3D: Convert the aromatic triketone to the 1,3,5-triethylcyclohexane shown below. ہوئےarrow_forwardIndicate how to find the energy difference between two levels in cm-1, knowing that its value is 2.5x10-25 joules.arrow_forward
- The gyromagnetic ratio (gamma) for 1H is 2.675x108 s-1 T-1. If the applied field is 1,409 T what will be the separation between nuclear energy levels?arrow_forwardChances Ad ~stract one 11. (10pts total) Consider the radical chlorination of 1,3-diethylcyclohexane depicted below. 4 • 6H total $4th total Statistical pro 21 total 2 H A 2H 래 • 4H totul < 3°C-H werkest bund - abstraction he leads to then mo fac a) (6pts) How many unique mono-chlorinated products can be formed and what are the structures for the thermodynamically and statistically favored products? рос 6 -વા J Number of Unique Mono-Chlorinated Products Thermodynamically Favored Product Statistically Favored Product b) (4pts) Draw the arrow pushing mechanism for the FIRST propagation step (p-1) for the formation of the thermodynamically favored product. Only draw the p-1 step. You do not need to include lone pairs of electrons. No enthalpy calculation necessary H H-Clarrow_forwardWhat is the lone pair or charge that surrounds the nitrogen here to give it that negative charge?arrow_forward
- Last Name, Firs Statifically more chances to abstract one of these 6H 11. (10pts total) Consider the radical chlorination of 1,3-diethylcyclohexane depicted below. 4 • 6H total $ 4th total 21 total 4H total ZH 2H Statistical H < 3°C-H werkst - product bund abstraction here leads to the mo favored a) (6pts) How many unique mono-chlorinated products can be formed and what are the structures for the thermodynamically and statistically favored products? Proclict 6 Number of Unique Mono-Chlorinated Products f Thermodynamically Favored Product Statistically Favored Product b) (4pts) Draw the arrow pushing mechanism for the FIRST propagation step (p-1) for the formation of the thermodynamically favored product. Only draw the p-1 step. You do not need to include lone pairs of electrons. No enthalpy calculation necessary 'H H-Cl Waterfoxarrow_forward2. (a) Many main group oxides form acidic solutions when added to water. For example solid tetraphosphorous decaoxide reacts with water to produce phosphoric acid. Write a balanced chemical equation for this reaction. (b) Calcium phosphate reacts with silicon dioxide and carbon graphite at elevated temperatures to produce white phosphorous (P4) as a gas along with calcium silicate (Silcate ion is SiO3²-) and carbon monoxide. Write a balanced chemical equation for this reaction.arrow_forwardI find the solution way too brief and unsatisfactory as it does not clearly explain the solution provided in the problem.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning  Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning
Nucleic acids - DNA and RNA structure; Author: MEDSimplified;https://www.youtube.com/watch?v=0lZRAShqft0;License: Standard YouTube License, CC-BY