
ORGANIC CHEMISTRY
5th Edition
ISBN: 9781259977596
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 21, Problem 21.5P
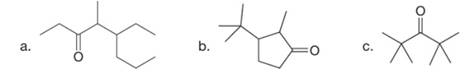
Give the IUPAC name for each
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Instructions: Complete the questions in the space provided. Show all your work
1. You are trying to determine the rate law expression for a reaction that you are completing at 25°C. You measure
the initial reaction rate and the starting concentrations of the reactions for 4 trials.
BrO³¯ (aq) + 5Br¯ (aq) + 6H* (aq) → 3Br₂ (l) + 3H2O (l)
Initial rate
Trial
[BrO3]
[H*]
[Br]
(mol/L)
(mol/L) | (mol/L)
(mol/L.s)
1
0.10
0.10
0.10
8.0
2
0.20
0.10
0.10
16
3
0.10
0.20
0.10
16
4
0.10
0.10
0.20
32
a.
Based on the above data what is the rate law expression?
b. Solve for the value of k (make sure to include proper units)
2. The proposed reaction mechanism is as follows:
i.
ii.
BrО¸¯ (aq) + H+ (aq) → HBrO3 (aq)
HBrO³ (aq) + H* (aq) → H₂BrO3* (aq)
iii.
H₂BrO³* (aq) + Br¯ (aq) → Br₂O₂ (aq) + H2O (l)
[Fast]
[Medium]
[Slow]
iv.
Br₂O₂ (aq) + 4H*(aq) + 4Br(aq) → 3Br₂ (l) + H2O (l)
[Fast]
Evaluate the validity of this proposed reaction. Justify your answer.
е.
Д
CH3
D*, D20
C.
NaOMe,
Br
Br
Chapter 21 Solutions
ORGANIC CHEMISTRY
Ch. 21 - Rank the following compounds in order of...Ch. 21 - Prob. 21.2PCh. 21 - Give the IUPAC name for each aldehyde.Ch. 21 - Prob. 21.4PCh. 21 - Give the IUPAC name for each ketone.Ch. 21 - Prob. 21.6PCh. 21 - Prob. 21.7PCh. 21 - The boiling point of is significantly higher than...Ch. 21 - Which carbonyl group in each pair absorbs at a...Ch. 21 - Problem 21.10 Draw the structure of all...
Ch. 21 - Prob. 21.11PCh. 21 - Prob. 21.12PCh. 21 - Prob. 21.13PCh. 21 - Prob. 21.14PCh. 21 - Problem 21.15 Draw the product of each...Ch. 21 - Prob. 21.16PCh. 21 - Problem 21.17 Draw the products of the following...Ch. 21 - Problem 21.18 Outline a synthesis of each Wittig...Ch. 21 - Problem 21.19 Draw the products (including...Ch. 21 - Problem 21.20 What starting materials are needed...Ch. 21 - Prob. 21.21PCh. 21 - Problem 21.22 The product formed when reacts with...Ch. 21 - Prob. 21.23PCh. 21 - Prob. 21.24PCh. 21 - Prob. 21.25PCh. 21 - Prob. 21.26PCh. 21 - Prob. 21.27PCh. 21 - Problem 21.28 Draw a stepwise mechanism for the...Ch. 21 - Problem 21.29 Draw the products of each...Ch. 21 - Problem 21.30 Label each compound as an acetal, a...Ch. 21 - Problem 21.31 Draw a stepwise mechanism for the...Ch. 21 - Problem 21.32 Draw the products of each...Ch. 21 - Problem 21.33 Safrole is a naturally occurring...Ch. 21 - Prob. 21.34PCh. 21 - Problem 21.35 How would you use a protecting group...Ch. 21 - Prob. 21.36PCh. 21 - Problem 21.37 Two naturally occurring compounds...Ch. 21 - Problem 21.38 Draw the products of each...Ch. 21 - Prob. 21.39PCh. 21 - Problem 21.40 (a) Give the IUPAC name for A and B....Ch. 21 - 21.41 Rank the following compounds in order of...Ch. 21 - Prob. 21.42PCh. 21 - 21.43 Give the IUPAC name for each compound.
a....Ch. 21 - 21.44 Give the structure corresponding to each...Ch. 21 - Prob. 21.45PCh. 21 - 21.46 Draw the products of each reaction.
a. e....Ch. 21 - Prob. 21.47PCh. 21 - 21.48 Draw all stereoisomers formed in each...Ch. 21 - Prob. 21.49PCh. 21 - What products are formed when each acetal is...Ch. 21 - Prob. 21.51PCh. 21 - Prob. 21.52PCh. 21 - Which compound forms the higher concentration of...Ch. 21 - Prob. 21.54PCh. 21 - Prob. 21.55PCh. 21 - Prob. 21.56PCh. 21 - Prob. 21.57PCh. 21 - Devise a synthesis of each alkene using a Wittig...Ch. 21 - Devise a synthesis of each compound from...Ch. 21 - Prob. 21.60PCh. 21 - Devise a synthesis of each compound from ethanol...Ch. 21 - Prob. 21.62PCh. 21 - Prob. 21.63PCh. 21 - 21.64 Draw a stepwise mechanism for the following...Ch. 21 - 21.65 Draw a stepwise mechanism f or the following...Ch. 21 - Prob. 21.66PCh. 21 - 21.67 Draw a stepwise mechanism for each...Ch. 21 - 21.68 Draw a stepwise mechanism for the following...Ch. 21 - Prob. 21.69PCh. 21 - Prob. 21.70PCh. 21 - Prob. 21.71PCh. 21 - Prob. 21.72PCh. 21 - 21.73 Although the carbonyl absorption of cyclic...Ch. 21 - 21.74 Use the and data to determine the...Ch. 21 - 21.75 A solution of acetone in ethanol in the...Ch. 21 - Compounds A and B have molecular formula ....Ch. 21 - 21.77 An unknown compound C of molecular formula ...Ch. 21 - 21.78 An unknown compound D exhibits a strong...Ch. 21 - Prob. 21.79PCh. 21 - -D-Glucose, a hemiacetal, can be converted to a...Ch. 21 - 21.81 Draw a stepwise mechanism for the following...Ch. 21 - Prob. 21.82PCh. 21 - 21.83 Draw a stepwise mechanism f or the...Ch. 21 - Prob. 21.84PCh. 21 - Prob. 21.85PCh. 21 - 21.86 Draw stepwise mechanism for the following...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please predict the products for each of the following reactions: 1.03 2. H₂O NaNH, 1. n-BuLi 2. Mel A H₂ 10 9 0 H2SO4, H₂O HgSO4 Pd or Pt (catalyst) B 9 2 n-BuLi ♡ D2 (deuterium) Lindlar's Catalyst 1. NaNH2 2. EtBr Na, ND3 (deuterium) 2. H₂O2, NaOH 1. (Sia)2BH с Darrow_forwardin the scope of ontario SCH4U grade 12 course, please show ALL workarrow_forwardIs the chemical reaction CuCl42-(green) + 4H2O <==> Cu(H2O)42+(blue) + 4Cl- exothermic or endothermic?arrow_forward
- If we react tetraethoxypropane with hydrazine, what is the product obtained (explain its formula). State the reason why the corresponding dialdehyde is not used.arrow_forwarddrawing, no aiarrow_forwardIf CH3COCH2CH(OCH3)2 (4,4-dimethoxy-2-butanone) and hydrazine react, two isomeric products are formed. State their structure and which will be the majority.arrow_forward
- + Reset Provide the correct IUPAC name for the compound shown here. 4-methylhept-2-ene (Z)- (E)- 1-6-5-2-3-4- cyclo iso tert- sec- di tri hept hex oct meth eth pent ane yne ene ylarrow_forward+ Provide the correct IUPAC name for the compound shown here. Reset H3C H H C CH3 CH-CH3 1-3-methylpent ene trans- cis- 5-6-3-1-2-4- tert- tri sec- di cyclo iso but pent hex meth prop eth yl ane ene yne ☑arrow_forwarddrawing, no aiarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY