
Concept explainers
(a)
Interpretation: The number of stereogenic centers present in
Concept introduction: Carbohydrates are naturally occurring compounds. Carbohydrates are polyhydroxy
Answer to Problem 21.39P
There are five stereogenic centers present in
Explanation of Solution
The stereogenic centers in
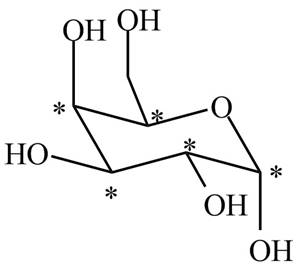
Figure 1
The stereogenic centers are marked by star. There are five stereogenic centers present in
There are five stereogenic centers present in
(b)
Interpretation: The hemiacetal carbon in
Concept introduction: Carbohydrates are naturally occurring compounds. Carbohydrates are polyhydroxy aldehydes and ketones. Galactose is a aldohexose as it contains six carbon atoms as well as an aldehyde functional group. The molecular formula of galactose
Aldehydes or ketones on reaction with one equivalent of alcohol form hemiacetal and on reaction with two equivalents of alcohol it forms acetals. This is nucleophilic addition reaction. These reactions takes place in presence of acids, commonly
Ethers contain only one alkoxy group on a carbon atom while acetals contain two alkoxy groups on a single carbon atom.
Hemiacetals contains one alkoxy group and one hydroxyl group attached to same carbon atom.
Answer to Problem 21.39P
The hemiacetal carbon in
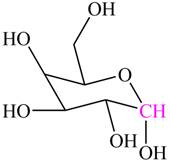
Explanation of Solution
The hemiacetal carbon in
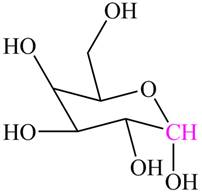
Figure 2
The highlighted carbon contains alkoxy group and hydroxyl group. Hence, this carbon is labeled as hemiacetal carbon.
The hemiacetal carbon in
(c)
Interpretation: The structure of
Concept introduction: Carbohydrates are naturally occurring compounds. Carbohydrates are polyhydroxy aldehydes and ketones. Galactose is a aldohexose as it contains six carbon atoms as well as an aldehyde functional group. The molecular formula of galactose
Answer to Problem 21.39P
The structure of
Explanation of Solution
In
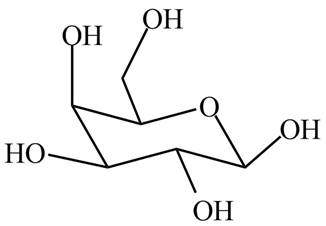
Figure 3
The structure of
(d)
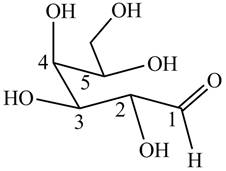
Interpretation: The structure of poly hydroxy aldehyde that cyclizes to
Concept introduction: Carbohydrates are naturally occurring compounds. Carbohydrates are polyhydroxy aldehydes and ketones. Galactose is a aldohexose as it contains six carbon atoms as well as an aldehyde functional group. The molecular formula of galactose
Aldehydes or ketones on reaction with one equivalent of alcohol form hemiacetal and on reaction with two equivalents of alcohol it forms acetals. This is nucleophilic addition reaction. These reactions takes place in presence of acids, commonly
In
Answer to Problem 21.39P
The structure of poly hydroxy aldehyde that cyclizes to
Explanation of Solution
The cyclization of poly hydroxy aldehyde results in the formation of hemiacetal. The hydroxyl group on

Figure 4
The structure of poly hydroxy aldehyde that cyclizes to
(e)
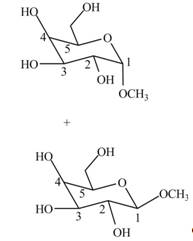
Interpretation: The products formed when
Concept introduction: Carbohydrates are naturally occurring compounds. Carbohydrates are polyhydroxy aldehydes and ketones. Galactose is a aldohexose as it contains six carbon atoms as well as an aldehyde functional group. The molecular formula of galactose
Aldehydes or ketones on reaction with one equivalent of alcohol form hemiacetal and on reaction with two equivalents of alcohol it forms acetals. This is nucleophilic addition reaction. These reactions takes place in presence of acids, commonly
Answer to Problem 21.39P
The products formed when
Explanation of Solution
Cyclic hemiacetals can be converted to acetals by treatment with alcohol in presence of acid. The hydroxyl group of hemiacetal is converted to alkoxy group. The
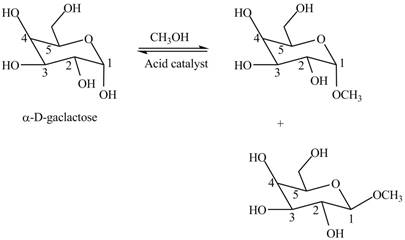
Figure 5
The products formed when
Want to see more full solutions like this?
Chapter 21 Solutions
ORGANIC CHEMISTRY
- Draw the Lewis structure for the polyatomic phosphite (PO¾³¯) a anion. Be sure to include all resonance structures that satisfy the octet rule. C I A [ ]¯arrow_forwardDecide whether these proposed Lewis structures are reasonable. proposed Lewis structure Is the proposed Lewis structure reasonable? Yes. :0: Cl C C1: 0=0: : 0 : : 0 : H C N No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* ☐ Yes. No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. ☐ No, it has the wrong number of valence electrons. The correct number is: ☐ No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* | * If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example, if two oxygen atoms don't satisfy the octet rule, enter "0,0".arrow_forwardDraw the Lewis structure for the polyatomic trisulfide anion. Be sure to include all resonance structures that satisfy the octet rule. с [ ] - Garrow_forward
- 1. Calculate the accurate monoisotopic mass (using all 1H, 12C, 14N, 160 and 35CI) for your product using the table in your lab manual. Don't include the Cl, since you should only have [M+H]*. Compare this to the value you see on the LC-MS printout. How much different are they? 2. There are four isotopic peaks for the [M+H]* ion at m/z 240, 241, 242 and 243. For one point of extra credit, explain what each of these is and why they are present. 3. There is a fragment ion at m/z 184. For one point of extra credit, identify this fragment and confirm by calculating the accurate monoisotopic mass. 4. The UV spectrum is also at the bottom of your printout. For one point of extra credit, look up the UV spectrum of bupropion on Google Images and compare to your spectrum. Do they match? Cite your source. 5. For most of you, there will be a second chromatographic peak whose m/z is 74 (to a round number). For one point of extra credit, see if you can identify this molecule as well and confirm by…arrow_forwardPlease draw, not just describe!arrow_forwardcan you draw each step on a piece of a paper please this is very confusing to mearrow_forward
- > Can the molecule on the right-hand side of this organic reaction be made in good yield from no more than two reactants, in one step, by moderately heating the reactants? esc ? A O O •If your answer is yes, then draw the reactant or reactants in the drawing area below. You can draw the reactants in any arrangement you like. • If your answer is no, check the box under the drawing area instead. olo 18 Ar Explanation Check BB Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibilityarrow_forwardName the structurearrow_forward> For each pair of substrates below, choose the one that will react faster in a substitution reaction, assuming that: 1. the rate of substitution doesn't depend on nucleophile concentration and 2. the products are a roughly 50/50 mixture of enantiomers. Substrate A Substrate B Faster Rate X CI (Choose one) (Choose one) CI Br Explanation Check Br (Choose one) C 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy A F10arrow_forward
- How to draw this mechanism for the foloowing reaction in the foto. thank youarrow_forwardPredict the major products of the following organic reaction: Some important notes: CN A? • Draw the major product, or products, of the reaction in the drawing area below. • If there aren't any products, because no reaction will take place, check the box below the drawing area instead. • Be sure to use wedge and dash bonds when necessary, for example to distinguish between major products that are enantiomers. No reaction. Explanation Check Click and drag to start drawing a structure. 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Centerarrow_forwardDraw the major product of the following reaction. Do not draw inorganic byproducts. H3PO4 OHarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





