
Fundamentals of Physics Extended
10th Edition
ISBN: 9781118230725
Author: David Halliday, Robert Resnick, Jearl Walker
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 20, Problem 43P
GO Figure 20-32 represents a Carnot engine that works between temperatures T1 = 400 K and T2= 150 K and drives a Carnot refrigerator that works between temperatures T3 = 325 K and T4 = 225 K. What is the ratio Q3/Q1?
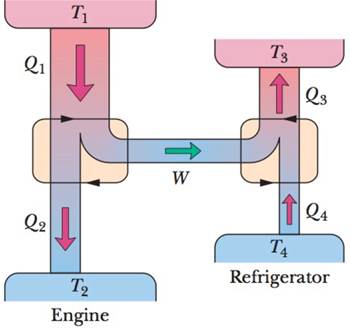
Figure 20-32 Problem 43.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
2
C01: Physical Quantities, Units and Measurementscobris alinu zotinUD TRO
Bendemeer Secondary School
Secondary Three Express Physics
Chpt 1: Physical Quantities, Unit and Measurements Assignment
Name: Chen ShiMan
loov neowled soria
25
( 03 ) Class: 3 Respect 6 Date: 2025.01.22
1
Which group consists only of scalar quantities?
ABCD
A
acceleration, moment and energy store
distance, temperature and time
length, velocity and current
mass, force and speed
B
D.
B
Which diagram represents the resultant vector of P and Q? lehtele
시
bas siqpeq olarist of beau eldeo qirie-of-qi
P
A
C
-B
qadmis
rle mengaib priwollot erT S
Quilons of qira ono mont aboog
eed indicator
yh from West
eril to Inioqbim srij
enisinoo MA
(6)
08 bas 8A aldao ni nolent or animaleb.gniweb slepe eld
260 km/h
D
1
D.
e
51
The figure gives the acceleration a versus time t for a particle moving along an x axis. The a-axis scale is set by as = 12.0 m/s². At t = -2.0
s, the particle's velocity is 11.0 m/s. What is its velocity at t = 6.0 s?
a (m/s²)
as
-2
0
2
t(s)
4
Two solid cylindrical rods AB and BC are welded together at B and loaded as shown. Knowing that the average normal stress must not
exceed 150 MPa in either rod, determine the smallest allowable values of the diameters d₁ and d2. Take P= 85 kN.
P
125 kN
B
125 kN
C
0.9 m
1.2 m
The smallest allowable value of the diameter d₁ is
The smallest allowable value of the diameter d₂ is
mm.
mm.
Chapter 20 Solutions
Fundamentals of Physics Extended
Ch. 20 - Point i in Fig. 20-19 represents the initial state...Ch. 20 - In lour experiments, blocks A and B, starting ill...Ch. 20 - A gas, confined to an insulated cylinder, is...Ch. 20 - An ideal monatomic gas at initial temperature T0...Ch. 20 - In four experiments, 2.5 mol of hydrogen gas...Ch. 20 - A box contains 100 atoms in a configuration that...Ch. 20 - Does the entropy per cycle increase, decrease, or...Ch. 20 - Three Carnot engines operate between temperature...Ch. 20 - An inventor claims to have invented four engines,...Ch. 20 - Does the entropy per cycle increase, decrease, or...
Ch. 20 - SSM Suppose 4.00 mol of an ideal gas undergoes a...Ch. 20 - An ideal gas undergoes a reversible isothermal...Ch. 20 - ILW A 2.50 mol sample of an ideal gas expands...Ch. 20 - How much energy must be transferred as heat for a...Ch. 20 - ILW Find a the energy absorbed as heat and b the...Ch. 20 - a What is the entropy change of a 12.0 g ice cube...Ch. 20 - ILW A 50.0 g block of copper whose temperature is...Ch. 20 - At very low temperatures, the molar specific heat...Ch. 20 - A 10 g ice cube at 10oC is placed in a lake whose...Ch. 20 - A 364 g block is put in contact with a thermal...Ch. 20 - SSM WWW In an experiment, 200 g of aluminum with a...Ch. 20 - A gas sample undergoes a reversible isothermal...Ch. 20 - In the irreversible process of Fig. 20-5, let the...Ch. 20 - Prob. 14PCh. 20 - A mixture of 1773 g of water and 227 g of ice is...Ch. 20 - GO An 8.0 g ice cube at -10C is put into a Thermos...Ch. 20 - Prob. 17PCh. 20 - GO A 2.0 mol sample of an ideal monatomic gas...Ch. 20 - Suppose 1.00 mol of a monatomic ideal gas is taken...Ch. 20 - Expand 1.00 mol of an monatomic gas initially at...Ch. 20 - GO Energy can be removed from water as heat at and...Ch. 20 - GO An insulated Thermos contains 130 g of water at...Ch. 20 - A Carnot engine whose low-temperature reservoir is...Ch. 20 - A Carnot engine absorbs 52 kJ as heat and exhausts...Ch. 20 - A Carnot engine has an efficiency of 22.0. It...Ch. 20 - In a hypothetical nuclear fusion reactor, the fuel...Ch. 20 - SSM WWW A Carnot engine operates between 235C and...Ch. 20 - In the first stage of a two-stage Carnot engine,...Ch. 20 - GO Figure 20-27 shows a reversible cycle through...Ch. 20 - A 500 W Carnot engine operates between...Ch. 20 - The efficiency of a particular car engine is 25...Ch. 20 - GO A Carnot engine is set up to produce a certain...Ch. 20 - SSM ILW Figure 20-29 shows a reversible cycle...Ch. 20 - GO An ideal gas 1.0 mol is the working substance...Ch. 20 - The cycle in Fig. 20-31 represents the operation...Ch. 20 - How much work must be done by a Carnot...Ch. 20 - SSM A heat pump is used to heal a building, The...Ch. 20 - The electric motor of a heat pump transfers energy...Ch. 20 - SSM A Carnot air conditioner lakes energy from the...Ch. 20 - To make ice, a freezer that is a reverse Carnot...Ch. 20 - ILW An air conditioner operating between 93F and...Ch. 20 - The motor in a refrigerator has a power of 200 W....Ch. 20 - GO Figure 20-32 represents a Carnot engine that...Ch. 20 - a During each cycle, a Carnot engine absorbs 750 J...Ch. 20 - Prob. 45PCh. 20 - A box contains N identical gas molecules equally...Ch. 20 - SSM WWW A box contains N gas molecules, Consider...Ch. 20 - Four particles are in the insulated box of Fig....Ch. 20 - A cylindrical copper rod of length 1.50 m and...Ch. 20 - Suppose 0.550 mol of an ideal gas is isothermally...Ch. 20 - Prob. 51PCh. 20 - Suppose 1.0 mol of a monatomic ideal gas initially...Ch. 20 - GO Suppose that a deep shaft were drilled in...Ch. 20 - What is the entropy change for 3.20 mol of an...Ch. 20 - A 600 g lump of copper at 80.0C is placed in 70.0...Ch. 20 - Figure 20-33 gives the force magnitude F versus...Ch. 20 - The temperature of 1.00 mol of a monatomic ideal...Ch. 20 - Repeat Problem 57, with the pressure now kept...Ch. 20 - SSM A 0.600 kg sample of water is initially ice at...Ch. 20 - A three-step cycle is undergone by 3.4 mol of an...Ch. 20 - An inventor has built an engine X and claims that...Ch. 20 - Suppose 2.00 mol of a diatomic gas is taken...Ch. 20 - A three-step cycle is undergone reversibly by 4.00...Ch. 20 - a A Carnot engine operates between a hot reservoir...Ch. 20 - A 2.00 mol diatomic gas initially at 300 K...Ch. 20 - An ideal refrigerator does 150 J of work to remove...Ch. 20 - Suppose that 260 J is conducted from a...Ch. 20 - An apparatus that liquefies helium is in a room...Ch. 20 - GO A brass rod is in thermal contact with a...Ch. 20 - A 45.0 g block of tungsten at 30.0C and a 25.0 g...Ch. 20 - Prob. 71PCh. 20 - Calculate the efficiency of a fossil-fuel power...Ch. 20 - SSM A Carnot refrigerator extracts 35.0 kJ as heat...Ch. 20 - A Carnot engine whose high-temperature reservoir...Ch. 20 - SSM System A of three particles and system B of...Ch. 20 - Figure 20-36 shows a Carnot cycle on a T-S...Ch. 20 - Find the relation between the efficiency of a...Ch. 20 - A Carnot engine has a power of 500 W. It operates...Ch. 20 - In a real refrigerator, the low-temperature coils...
Additional Science Textbook Solutions
Find more solutions based on key concepts
3. What is free-fall, and why does it make you weightless? Briefly describe why astronauts are weightless in th...
The Cosmic Perspective (8th Edition)
The number of named species is about ________, but the actual number of species on Earth is estimated to be abo...
Biology: Life on Earth with Physiology (11th Edition)
Choose the best answer to each of the following. Explain your reasoning. 1.Measurement of how orbital aur vital...
Cosmic Perspective Fundamentals
The bioremediation process shown in the photograph is used to remove benzene and other hydrocarbons from soil c...
Microbiology: An Introduction
8.63 Two flasks of equal volume and at the same temperature contain different gases. One flask contains 10.0 g ...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Calculate the lattice energy of CaCl2 using a Born-Haber cycle and data from Appendices F and L and Table 7.5. ...
Chemistry & Chemical Reactivity
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Westros, from Game of Thrones, has an area of approximately 6.73⋅106 miles26.73⋅106miles2. Convert the area of Westros to km2 where 1.00 mile = 1.609 km.arrow_forwarda) What is the lenght of x? b) Findθ c) Find ϕarrow_forwardA surveyor measures the distance across a straight river by the following method: Starting directly across from a tree on the opposite bank, he walks x = 97.7 m along the riverbank to establish a baseline. Then he sights across to the tree. The angle from his baseline to the tree is θ = 33.0 °. How wide is the river?arrow_forward
- A small turtle moves at a speed of 697. furlong/fortnight. Find the speed of the turtle in centimeters per second. Note that 1.00 furlong = 220. yards, 1.00 yard = 3.00 feet, 1.00 foot = 12.0 inches, 1.00 inch = 2.54 cm, and 1.00 fortnight = 14.0 days.arrow_forwardThe landmass of Sokovia lifted in the air in Avengers: Age of Ultron had a volume of about 1.98 km3. What volume is that in m3?arrow_forwardA fathom is a unit of length, usually reserved for measuring the depth of water. A fathom is exactly 6.00 ft in length. Take the distance from Earth to the Moon to be 252,000 miles, and use the given approximation to find the distance in fathoms. 1 mile = 5280 ft. (Answer in sig fig.)arrow_forward
- No chatgpt pls will upvotearrow_forwardOne of the earliest video games to have a plot, Zork, measured distances in “Bloits” where 1 Bloit was defined as the distance the king’s favorite pet could run in one hour, 1,090 m. In the same game the king has a statue made that is 9.00 Bloits high. What is this in meters?arrow_forwardNo chatgpt pls will upvotearrow_forward
- No chatgpt pls will upvotearrow_forwardDefination of voltagearrow_forwardAt point A, 3.20 m from a small source of sound that is emitting uniformly in all directions, the intensity level is 58.0 dB. What is the intensity of the sound at A? How far from the source must you go so that the intensity is one-fourth of what it was at A? How far must you go so that the sound level is one-fourth of what it was at A?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
 Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning


Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
The Second Law of Thermodynamics: Heat Flow, Entropy, and Microstates; Author: Professor Dave Explains;https://www.youtube.com/watch?v=MrwW4w2nAMc;License: Standard YouTube License, CC-BY