
Fundamentals of Physics Extended
10th Edition
ISBN: 9781118230725
Author: David Halliday, Robert Resnick, Jearl Walker
Publisher: Wiley, John & Sons, Incorporated
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 20, Problem 35P
The cycle in Fig. 20-31 represents the operation of a gasoline internal combustion engine. Volume V3 = 4.00V1. Assume the gasoline-air intake mixture is an ideal gas with γ = 1.30. What are the ratios (a) T2/T1,(b) T3/T1, (c) T4/T1, (d) p3/p1, and (e) p4/p1? (f) What is the engine efficiency?
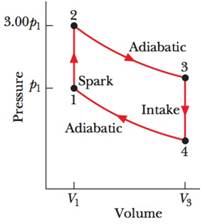
Figure 20-31 Problem 35.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
As I carry a box up a flight of stairs, am I doing positive work or negative work on the box? Provide a mathematical explanation.
As a ball falls under the influence of gravity, does gravity do positive work or negative work? Provide a mathematical explanation.
Under what circumstances is it bad to describe kinetic energy as k = 1/2mv^2
Chapter 20 Solutions
Fundamentals of Physics Extended
Ch. 20 - Point i in Fig. 20-19 represents the initial state...Ch. 20 - In lour experiments, blocks A and B, starting ill...Ch. 20 - A gas, confined to an insulated cylinder, is...Ch. 20 - An ideal monatomic gas at initial temperature T0...Ch. 20 - In four experiments, 2.5 mol of hydrogen gas...Ch. 20 - A box contains 100 atoms in a configuration that...Ch. 20 - Does the entropy per cycle increase, decrease, or...Ch. 20 - Three Carnot engines operate between temperature...Ch. 20 - An inventor claims to have invented four engines,...Ch. 20 - Does the entropy per cycle increase, decrease, or...
Ch. 20 - SSM Suppose 4.00 mol of an ideal gas undergoes a...Ch. 20 - An ideal gas undergoes a reversible isothermal...Ch. 20 - ILW A 2.50 mol sample of an ideal gas expands...Ch. 20 - How much energy must be transferred as heat for a...Ch. 20 - ILW Find a the energy absorbed as heat and b the...Ch. 20 - a What is the entropy change of a 12.0 g ice cube...Ch. 20 - ILW A 50.0 g block of copper whose temperature is...Ch. 20 - At very low temperatures, the molar specific heat...Ch. 20 - A 10 g ice cube at 10oC is placed in a lake whose...Ch. 20 - A 364 g block is put in contact with a thermal...Ch. 20 - SSM WWW In an experiment, 200 g of aluminum with a...Ch. 20 - A gas sample undergoes a reversible isothermal...Ch. 20 - In the irreversible process of Fig. 20-5, let the...Ch. 20 - Prob. 14PCh. 20 - A mixture of 1773 g of water and 227 g of ice is...Ch. 20 - GO An 8.0 g ice cube at -10C is put into a Thermos...Ch. 20 - Prob. 17PCh. 20 - GO A 2.0 mol sample of an ideal monatomic gas...Ch. 20 - Suppose 1.00 mol of a monatomic ideal gas is taken...Ch. 20 - Expand 1.00 mol of an monatomic gas initially at...Ch. 20 - GO Energy can be removed from water as heat at and...Ch. 20 - GO An insulated Thermos contains 130 g of water at...Ch. 20 - A Carnot engine whose low-temperature reservoir is...Ch. 20 - A Carnot engine absorbs 52 kJ as heat and exhausts...Ch. 20 - A Carnot engine has an efficiency of 22.0. It...Ch. 20 - In a hypothetical nuclear fusion reactor, the fuel...Ch. 20 - SSM WWW A Carnot engine operates between 235C and...Ch. 20 - In the first stage of a two-stage Carnot engine,...Ch. 20 - GO Figure 20-27 shows a reversible cycle through...Ch. 20 - A 500 W Carnot engine operates between...Ch. 20 - The efficiency of a particular car engine is 25...Ch. 20 - GO A Carnot engine is set up to produce a certain...Ch. 20 - SSM ILW Figure 20-29 shows a reversible cycle...Ch. 20 - GO An ideal gas 1.0 mol is the working substance...Ch. 20 - The cycle in Fig. 20-31 represents the operation...Ch. 20 - How much work must be done by a Carnot...Ch. 20 - SSM A heat pump is used to heal a building, The...Ch. 20 - The electric motor of a heat pump transfers energy...Ch. 20 - SSM A Carnot air conditioner lakes energy from the...Ch. 20 - To make ice, a freezer that is a reverse Carnot...Ch. 20 - ILW An air conditioner operating between 93F and...Ch. 20 - The motor in a refrigerator has a power of 200 W....Ch. 20 - GO Figure 20-32 represents a Carnot engine that...Ch. 20 - a During each cycle, a Carnot engine absorbs 750 J...Ch. 20 - Prob. 45PCh. 20 - A box contains N identical gas molecules equally...Ch. 20 - SSM WWW A box contains N gas molecules, Consider...Ch. 20 - Four particles are in the insulated box of Fig....Ch. 20 - A cylindrical copper rod of length 1.50 m and...Ch. 20 - Suppose 0.550 mol of an ideal gas is isothermally...Ch. 20 - Prob. 51PCh. 20 - Suppose 1.0 mol of a monatomic ideal gas initially...Ch. 20 - GO Suppose that a deep shaft were drilled in...Ch. 20 - What is the entropy change for 3.20 mol of an...Ch. 20 - A 600 g lump of copper at 80.0C is placed in 70.0...Ch. 20 - Figure 20-33 gives the force magnitude F versus...Ch. 20 - The temperature of 1.00 mol of a monatomic ideal...Ch. 20 - Repeat Problem 57, with the pressure now kept...Ch. 20 - SSM A 0.600 kg sample of water is initially ice at...Ch. 20 - A three-step cycle is undergone by 3.4 mol of an...Ch. 20 - An inventor has built an engine X and claims that...Ch. 20 - Suppose 2.00 mol of a diatomic gas is taken...Ch. 20 - A three-step cycle is undergone reversibly by 4.00...Ch. 20 - a A Carnot engine operates between a hot reservoir...Ch. 20 - A 2.00 mol diatomic gas initially at 300 K...Ch. 20 - An ideal refrigerator does 150 J of work to remove...Ch. 20 - Suppose that 260 J is conducted from a...Ch. 20 - An apparatus that liquefies helium is in a room...Ch. 20 - GO A brass rod is in thermal contact with a...Ch. 20 - A 45.0 g block of tungsten at 30.0C and a 25.0 g...Ch. 20 - Prob. 71PCh. 20 - Calculate the efficiency of a fossil-fuel power...Ch. 20 - SSM A Carnot refrigerator extracts 35.0 kJ as heat...Ch. 20 - A Carnot engine whose high-temperature reservoir...Ch. 20 - SSM System A of three particles and system B of...Ch. 20 - Figure 20-36 shows a Carnot cycle on a T-S...Ch. 20 - Find the relation between the efficiency of a...Ch. 20 - A Carnot engine has a power of 500 W. It operates...Ch. 20 - In a real refrigerator, the low-temperature coils...
Additional Science Textbook Solutions
Find more solutions based on key concepts
3. Which of the following is a major functional characteristic of all organisms? (a) movement, (b) growth (c) m...
Human Anatomy & Physiology (Marieb, Human Anatomy & Physiology) Standalone Book
Describe how the barometric pressure will change at Point F as the midlatitude cyclone moves eastward. ________...
Applications and Investigations in Earth Science (9th Edition)
How do food chains and food webs differ? Which is the more accurate representation of feeding relationships in ...
Biology: Life on Earth (11th Edition)
7. Which bones form via intramembranous ossification?
a. Irregular bones
b. Certain flat bones
c. Long bones
d....
Human Anatomy & Physiology (2nd Edition)
Chemical equations must be used to describe the difference between sugar melting and sugar decomposing. Concept...
Living By Chemistry: First Edition Textbook
Explain all answers clearly, using complete sentences and proper essay structure if needed. An asterisk (*) des...
Cosmic Perspective Fundamentals
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- No chatgpt pls will upvotearrow_forwardAir temperature of 37 °C increases swimming pool temperature of 2.55 °C. What is the fraction of the water in the pool must evaporate during this time to carry enough energy to keep the temperature of the pool constant? 4186 J/(kg°C) = specific heat of water 2,430,000 (2.43 x 106) J/kg = latent heat of vaporization for the water in the pool.arrow_forwardThe iceberg requires 7.4 x 1020 Joules of energy to melt it completely. It absorbs energy from the Sun at a constant average rate of 88 Watts/m2. The total surface area of iceberg exposed to the sunlight is 12 billion (1.2 x 1010) square meters. How long will it take for sunlight to melt the entire iceberg in yearsarrow_forward
- 1.0 kg block of ice to melt in the kitchen. The temperature in the kitchen is 31 °C. The ice starts out at 0 °C and takes an hour to melt and reach the same temperature as the surrounding room (31 °C). How much heat does the 1.0 kg of ice/water absorb from the room as it melts and heats up to 31 °C in Joules absorbed? Latent heat of fusion for water/ice is 334,000 J/kg Specific heat of water is 4186 J/kg°Carrow_forward5.84 If the coefficient of static friction between a table and a uni- form, massive rope is μ, what fraction of the rope can hang over the edge of the table without the rope sliding? 5.97 Block A, with weight Figure P5.97 3w, slides down an inclined plane S of slope angle 36.9° at a constant speed while plank B, with weight w, rests on top of A. The plank is attached by a cord to the wall (Fig. P5.97). (a) Draw a diagram of all the forces acting on block A. (b) If the coefficient of kinetic friction is the same between A and B and between S and A, determine its value. 36.9° 1arrow_forwardNo chatgpt pls will upvotearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
 Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning


Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning
The Second Law of Thermodynamics: Heat Flow, Entropy, and Microstates; Author: Professor Dave Explains;https://www.youtube.com/watch?v=MrwW4w2nAMc;License: Standard YouTube License, CC-BY