
Organic Chemistry
7th Edition
ISBN: 9780321803221
Author: Paula Y. Bruice
Publisher: Prentice Hall
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 56P
a. Rank the following alcohols from strongest to weakest acid.
b. Explain the relative acidities.
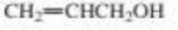
CH3CH2CH2OH
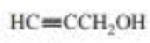
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Q7.
a. Draw the line-bond structure of the major product for the following reaction, if a reaction
occurs, assume monohalogenation.
b. Calculate the product ratios using the following information (hint: use the number of
hydrogens in each category present to calculate the ratios).
Chlorination: 1° Reactivity=1
2° Reactivity=4
Heat
+ Cl2
3° Reactivity=5
Please correct answer and don't use hand rating and don't use Ai solution
Q10: Alkane halogenation
a. Give the name and structures of the five isomeric hexanes.
Page 4 of 5
Chem 0310 Organic Chemistry 1 Recitations
b. For each isomer, give all the free radical monochlorination and monobromination products
that are structurally isomeric.
Chapter 2 Solutions
Organic Chemistry
Ch. 2.1 - Which of the following are not acids? CH3COOH CO2...Ch. 2.1 - Draw the products of the addbase renc1 ion when a....Ch. 2.1 - a. What is the conjugate acid of each or the...Ch. 2.2 - a. Which is a stronger acid: one with a pKa of 5.2...Ch. 2.2 - An acid has a Ka of 4.53 106 in water. What is...Ch. 2.2 - Prob. 6PCh. 2.2 - Antacids are compounds that neutralize stomach...Ch. 2.2 - Are the following body fluids acidic or basic? a....Ch. 2.3 - Draw the conjugate acid of each of the following:...Ch. 2.3 - a. Write an equation showing CH3OH reacting as an...
Ch. 2.3 - Estimate the pKa values of the following...Ch. 2.3 - a. Which is a stronger base: CH3COO or HCOO? (The...Ch. 2.3 - Using the pKa values in Section 2.3, rank the...Ch. 2.4 - Prob. 14PCh. 2.5 - a. For each of the acid-base reactions in Section...Ch. 2.5 - Ethyne has a pKa value of 25, water has a pKa...Ch. 2.5 - Which of the following bases can remove a proton...Ch. 2.5 - Calculate the equilibrium constant for the...Ch. 2.6 - Rank the ions (CH3, NH2, HO, and F) from most...Ch. 2.6 - Rank the carbanions shown in the margin from most...Ch. 2.6 - Which is the stronger acid?Ch. 2.6 - Prob. 22PCh. 2.6 - Rank the halide ions (F, Cl, Br, and l) from...Ch. 2.6 - a. Which is more electronegative, oxygen or...Ch. 2.6 - Which is a stronger acid? a. HCl or HBr b....Ch. 2.6 - a. Which of the halide ions (F, Cl, Br, and l) is...Ch. 2.6 - Prob. 27PCh. 2.7 - What is a stronger acid? a. CH3OCH2CH2OH or...Ch. 2.7 - Prob. 29PCh. 2.7 - What is a stronger base?Ch. 2.8 - Fosamax (shown on the previous page) has six...Ch. 2.8 - Which is a stronger acid? Why?Ch. 2.8 - Prob. 34PCh. 2.9 - Using the table of pKa values given in Appendix I,...Ch. 2.10 - Prob. 36PCh. 2.10 - As long as the pH is not less than _______, at...Ch. 2.10 - A naturally occurring amino acid such as alanine...Ch. 2.10 - For each of the following compounds, indicate the...Ch. 2.11 - Write the equation that shows how a buffer made by...Ch. 2.12 - Draw the products of the following react ions. Use...Ch. 2.12 - What product are formed when each of the following...Ch. 2 - a. Rank the following alcohols from strongest to...Ch. 2 - Which is a stronger base? a. HS or HO b. CH3O or...Ch. 2 - According to the explanations by Lewis, if a...Ch. 2 - a. Rank the following carboxylic acids from...Ch. 2 - Prob. 51PCh. 2 - For the following compound. a. draw its conjugate...Ch. 2 - Rank the following compounds from strongest to...Ch. 2 - Prob. 54PCh. 2 - Prob. 55PCh. 2 - a. Rank the following alcohols from strongest to...Ch. 2 - For each compound, indicate the atom that is most...Ch. 2 - a. Given the Ka values, estimate the pKa value of...Ch. 2 - A single bond between two carbons with different...Ch. 2 - Tenormin, a member of the group of drugs known as...Ch. 2 - From which of the following compounds can HO...Ch. 2 - a. For each of the following pairs of reactions,...Ch. 2 - Prob. 63PCh. 2 - Which is a stronger acid? a. b. c. d.Ch. 2 - Prob. 65PCh. 2 - Given that pH+ pOH = 14 and that the concentration...Ch. 2 - How could you separate a mixture of the following...Ch. 2 - Prob. 68PCh. 2 - a. If an add with a pKa of 5.3 is in an aqueous...Ch. 2 - Calculate the pH values of the following...Ch. 2 - Prob. 1PCh. 2 - Prob. 2PCh. 2 - Prob. 3PCh. 2 - Which of the reactions in Problem 3 favor...Ch. 2 - Prob. 5PCh. 2 - Prob. 6PCh. 2 - Prob. 7PCh. 2 - Which is the stronger acid? a. ClCH2CH2OH or...Ch. 2 - Prob. 9PCh. 2 - Prob. 10PCh. 2 - Which is a more stable base? Remembering that the...Ch. 2 - Which is the Stronger acid?Ch. 2 - Prob. 13PCh. 2 - a. Draw the structure of (CH3COOH (pKa = 4.7) at...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Q9. The insecticide DDT (in the box below) is useful in controlling mosquito populations and has low toxicity to humans, but is dangerous to birds and fish. Hoping to alleviate the dangers, little Johnny Whizbang, an aspiring chemist, proposes a new version of DDT ("Bromo-DDT") and shows his synthesis to his boss. Will Johnny Whizbang's synthesis work? Or will he be fired? Assume there is an excess of bromine and polybrominated products can be separated. Explain why. CH3 Br2, light CBR3 ok-ok Br Br Br Br CI "Bromo-DDT" CCl 3 DDT (dichlorodiphenyltrichloroethane) CIarrow_forwardDifferentiate the terms Monotectic, Eutectic, Eutectoid, Peritectic, Peritectoid.arrow_forwardQ5. Predict the organic product(s) for the following transformations. If no reaction will take place (or the reaction is not synthetically useful), write "N.R.". Determine what type of transition state is present for each reaction (think Hammond Postulate). I Br₂ CH3 F2, light CH3 Heat CH3 F₂ Heat Br2, light 12, light CH3 Cl2, lightarrow_forward
- a. For the following indicated bonds, rank them in order of decreasing AH° for homolytic cleavage. Based on your answer, which bond would be most likely to break homolytically? (a) (c) H3C CH3 .CH3 CH3 CH3 (b) Page 1 of 5 Chem 0310 Organic Chemistry 1 Recitations b. Draw all the possible radical products for 2-methylbutane, and determine which bond is most likely to be broken.arrow_forwardA 5-m³ rigid tank contains 5 kg of water at 100°C. Determine (a) the pressure, (b) the total enthalpy, and (c) the mass of each phase of water.arrow_forwardQ8. Draw the mechanism for this halogenation reaction. Show all steps including initiation, propagation, and recombination. Cl₂, hv CI Br Br2, hv, heatarrow_forward
- Q6. Given the following alkanes, draw the most likely product to form upon monohalogenation with Br2 (keep in mind that this may not be the only product to form though). If the reaction was performed with Cl2 would there be more or less selectivity in the desired product formation? Why? (a) (b) (c)arrow_forwardQ4. Radicals a. For the following indicated bonds, rank them in order of decreasing AH° for homolytic cleavage. Based on your answer, which bond would be most likely to break homolytically? (c) CH3 CH3 H3C CH3 (a) CH3 (b)arrow_forwardQ1. (a) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH3. Use curved arrows to show the electron movement. (b) Draw equations for homolytic and heterolytic cleavages of the N-H bond in NH4*. Use curved arrows to show the electron movement.arrow_forward
- ohing Quantitative Relationships 425 The specific heats and atomic masses of 20 of the elements are given in the table below. Use a graphical method to determine if there is a relationship between specific heat and the atomic mass. a. b. C. d. e. If your graphs revealed relationship between specific heat and atomic revealed a mathematical mass, write down an equation for the relationship. Comment on the usefulness of the determination of specific heat as a method for identifying an element. Would specific heat alone give you much confidence with regard to the identity of the element? If you think measurement of another property would be needed to support an identification, what property would you measure and why? The elements listed in the table are all selected metals. The values for nitrogen, oxygen, fluorine and neon are 1.040, 0.918, 0.824 and 1.030 J/g K respectively. Do these elements fit your equation? element atomic mass specific heat (almol) (Jig K) magnesium 24.305 1.023…arrow_forwardPlease correct answer and don't use hand rating and don't use Ai solutionarrow_forwardNonearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY