
OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card for McMurry's Organic Chemistry, 9th
9th Edition
ISBN: 9781305671874
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 19.13, Problem 22P
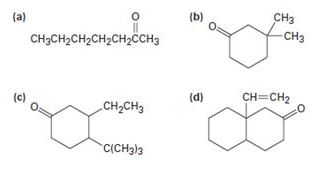
How might conjugate addition reactions of lithium diorganocopper reagents be used to synthesize the following compounds?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Show reaction mechanism. Don't give Ai generated solution
Describe some isomerism that carboranes have.
Indicate an isomerism that carboranes present.
Chapter 19 Solutions
OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card for McMurry's Organic Chemistry, 9th
Ch. 19.1 - Prob. 1PCh. 19.1 - Draw structures corresponding to the following...Ch. 19.2 - Prob. 3PCh. 19.2 - How would you carry out the following reactions?...Ch. 19.4 - Treatment of an aldehyde or ketone with cyanide...Ch. 19.4 - p-Nitrobenzaldehyde is more reactive toward...Ch. 19.5 - Prob. 7PCh. 19.5 - The oxygen in water is primarily (99.8) 16O, but...Ch. 19.6 - Prob. 9PCh. 19.8 - Show the products you would obtain by...
Ch. 19.8 - Prob. 11PCh. 19.8 - Prob. 12PCh. 19.9 - Prob. 13PCh. 19.10 - Prob. 14PCh. 19.10 - Prob. 15PCh. 19.11 - What carbonyl compound and what phosphorus ylide...Ch. 19.11 - -Carotene, a yellow food-coloring agent and...Ch. 19.12 - Prob. 18PCh. 19.12 - Prob. 19PCh. 19.13 - Prob. 20PCh. 19.13 - Treatment of 2-cyclohexenone with HCN/KCN yields a...Ch. 19.13 - How might conjugate addition reactions of lithium...Ch. 19.14 - How might you use IR spectroscopy to determine...Ch. 19.14 - Prob. 24PCh. 19.14 - Prob. 25PCh. 19.14 - Prob. 26PCh. 19.SE - Each of the following substances can be prepared...Ch. 19.SE - Prob. 28VCCh. 19.SE - Prob. 29VCCh. 19.SE - Prob. 30MPCh. 19.SE - Prob. 31MPCh. 19.SE - Prob. 32MPCh. 19.SE - Prob. 33MPCh. 19.SE - Prob. 34MPCh. 19.SE - Prob. 35MPCh. 19.SE - It is not uncommon for organic chemists to prepare...Ch. 19.SE - Prob. 37MPCh. 19.SE - Prob. 38MPCh. 19.SE - Prob. 39MPCh. 19.SE - Prob. 40MPCh. 19.SE - Aldehydes and ketones react with thiols to yield...Ch. 19.SE - Prob. 42MPCh. 19.SE - When cyclohexanone is heated in the presence of a...Ch. 19.SE - Prob. 44MPCh. 19.SE - The Meerwein-Ponndorf-Verley reaction involves...Ch. 19.SE - Propose a mechanism to account for the formation...Ch. 19.SE - Prob. 47MPCh. 19.SE - Prob. 48MPCh. 19.SE - Treatment of an , -unsaturated ketone with basic...Ch. 19.SE - Prob. 50MPCh. 19.SE - Prob. 51MPCh. 19.SE - Prob. 52MPCh. 19.SE - Prob. 53MPCh. 19.SE - Prob. 54APCh. 19.SE - Draw and name the seven aldehydes and ketones with...Ch. 19.SE - Give IUPAC names for the following compounds:Ch. 19.SE - Draw structures of compounds that fit the...Ch. 19.SE - Predict the products of the reaction of (1)...Ch. 19.SE - Show how you might use a Wittig reaction to...Ch. 19.SE - How would you use a Grignard reaction on an...Ch. 19.SE - Prob. 61APCh. 19.SE - Prob. 62APCh. 19.SE - How would you synthesize the following substances...Ch. 19.SE - Carvone is the major constituent of spearmint oil....Ch. 19.SE - How would you synthesize the following compounds...Ch. 19.SE - At what position would you expect to observe IR...Ch. 19.SE - Acidcatalyzed dehydration of...Ch. 19.SE - Choose the structure that best fits the IR...Ch. 19.SE - Propose structures for molecules that meet the...Ch. 19.SE - Prob. 70APCh. 19.SE - Prob. 71APCh. 19.SE - When 4hydroxybutanal is treated with methanol in...Ch. 19.SE - Prob. 73APCh. 19.SE - Prob. 74APCh. 19.SE - Prob. 75APCh. 19.SE - Prob. 76APCh. 19.SE - Prob. 77APCh. 19.SE - Tamoxifen is a drug used in the treatment of...Ch. 19.SE - Compound A, MW 86, shows an IR absorption at 1730...Ch. 19.SE - Compound B is isomeric with A (Problem 19-79) and...Ch. 19.SE - The 1HNMR spectrum shown is that of a compound...Ch. 19.SE - Prob. 82APCh. 19.SE - Propose structures for ketones or aldehydes that...Ch. 19.SE - Prob. 84APCh. 19.SE - Prob. 85APCh. 19.SE - The proton and carbon NMR spectra for each of...Ch. 19.SE - The proton NMR spectrum for a compound with...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Transmitance 3. Which one of the following compounds corresponds to this IR spectrum? Point out the absorption band(s) that helped you decide. OH H3C OH H₂C CH3 H3C CH3 H3C INFRARED SPECTRUM 0.8- 0.6 0.4- 0.2 3000 2000 1000 Wavenumber (cm-1) 4. Consider this compound: H3C On the structure above, label the different types of H's as A, B, C, etc. In table form, list the labeled signals, and for each one state the number of hydrogens, their shifts, and the splitting you would observe for these hydrogens in the ¹H NMR spectrum. Label # of hydrogens splitting Shift (2)arrow_forwardNonearrow_forwardDraw the Lewis structure of C2H4Oarrow_forward
- a) 5. Circle all acidic (and anticoplanar to the Leaving group) protons in the following molecules, Solve these elimination reactions, and identify the major and minor products where appropriate: 20 points + NaOCH3 Br (2 productarrow_forwardNonearrow_forwardDr. Mendel asked his BIOL 260 class what their height was and what their parent's heights were. He plotted that data in the graph below to determine if height was a heritable trait. A. Is height a heritable trait? If yes, what is the heritability value? (2 pts) B. If the phenotypic variation is 30, what is the variation due to additive alleles? (2 pts) Offspring Height (Inches) 75 67.5 60 52.5 y = 0.9264x + 4.8519 55 60 65 MidParent Height (Inches) 70 75 12pt v V Paragraph B IUA > AT2 v Varrow_forward
- Experiment: Each team will be provided with 5g of a mixture of acetanilide and salicylic acid. You will divide it into three 1.5 g portions in separate 125 mL Erlenmeyer flasks savıng some for melting point analysis. Dissolve the mixture in each flask in ~60mL of DI water by heating to boiling on a hotplate. Take the flasks off the hotplate once you have a clear solution and let them stand on the bench top for 5 mins and then allow them to cool as described below. Sample A-Let the first sample cool slowly to room temperature by letting it stand on your lab bench, with occasional stirring to promote crystallization. Sample B-Cool the second sample 1n a tap-water bath to 10-15 °C Sample C-Cool the third sample in an ice-bath to 0-2 °C Results: weight after recrystalization and melting point temp. A=0.624g,102-115° B=0.765g, 80-105° C=1.135g, 77-108 What is the percent yield of A,B, and C.arrow_forwardRel. Intensity Q 1. Which one of the following is true of the compound whose mass spectrum is shown here? Explain how you decided. 100 a) It contains chlorine. b) It contains bromine. c) It contains neither chlorine nor bromine. 80- 60- 40- 20- 0.0 0.0 TT 40 80 120 160 m/z 2. Using the Table of IR Absorptions how could you distinguish between these two compounds in the IR? What absorbance would one compound have that the other compound does not? HO CIarrow_forwardIllustrate reaction mechanisms of alkenes with water in the presence of H2SO4, detailing each step of the process. Please show steps of processing. Please do both, I will thumb up for sure #1 #3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole
Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License