
a)
Interpretation:
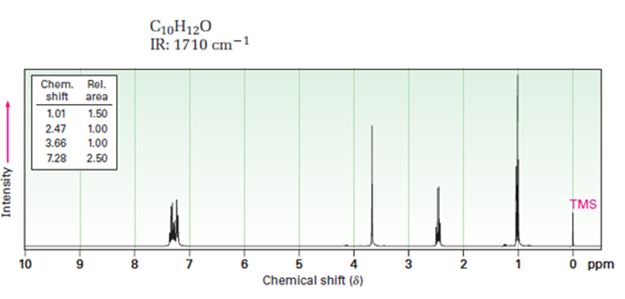
A structure for ketone or aldehyde with the following descriptions is to be proposed.
C10H12O, IR: 1710 cm-1; 1HNMR: 1.01 δ (Rel.area=1.50), 2.47 δ (Rel.area=1.00), 3.66 δ (Rel.area=1.00), 7.28 δ (Rel.area=2.50).
Concept introduction:
In 1HNMR the aldehyde protons absorb near 10 δ with a coupling constant, J=3Hz. Hydrogens on the carbon next to aldehyde group absorb near 2.0-2.3 δ. Methyl ketones show a sharp three proton singlet near 2.1 δ.
To propose:
A structure for ketone or aldehyde with the following descriptions.
C10H12O, IR: 1710 cm-1; 1HNMR: 1.01 δ (Rel.area=1.50), 2.47 δ (Rel.area=1.00), 3.66 δ (Rel.area=1.00), 7.28 δ (Rel.area=2.50).
b)
Interpretation:
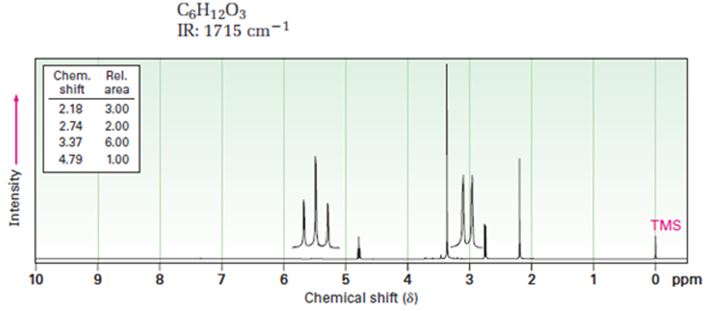
A structure for ketone or aldehyde with the following descriptions is to be proposed.
C6H12O3, IR: 1715 cm-1; 1HNMR: 2.18 δ (Rel.area=3.00), 2.74 δ (Rel.area=2.00), 3.37 δ (Rel.area=6.00), 4.79 δ (Rel.area=1.00).
Concept introduction:
Aldehydes and ketones show a strong absorption band in IR from 1660-1770 cm-1. Aldehydes show two characteristic C-H absorptions between 2700-2760 cm-1 and 2800-2860 cm-1. Saturated aldehydes absorb near 1730 cm-1 while aromatic aldehydes and α, β-unsaturated aldehydes absorb near 1705 cm-1. Saturated ketones and cyclohexanones absorb near 1715 cm-1 while aromatic ketones and α, β-unsaturated ketones absorb near 1685-1690 cm-1. Cyclopentanones absorb around 1750 cm-1.
In 1HNMR the aldehyde protons absorb near 10 δ with a coupling constant, J=3Hz. Hydrogens on the carbon next to aldehyde group absorb near 2.0-2.3 δ. Methyl ketones show a sharp three proton singlet near 2.1 δ.
To propose:
A structure for ketone or aldehyde with the following descriptions.
C6H12O3, IR: 1715 cm-1; 1HNMR: 2.18 δ (Rel.area=3.00), 2.74 δ (Rel.area=2.00), 3.37 δ (Rel.area=6.00), 4.79 δ (Rel.area=1.00).
Trending nowThis is a popular solution!

Chapter 19 Solutions
OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card for McMurry's Organic Chemistry, 9th
- A covalent bond is the result of the a) b) c) d) e) overlap of two half-filled s orbitals overlap of a half-filled s orbital and a half-filled p orbital overlap of two half-filled p orbitals along their axes parallel overlap of two half-filled parallel p orbitals all of the abovearrow_forwardCan the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left? starting material target If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area. Be sure you follow the standard ALEKS rules for submitting syntheses. + More... Note for advanced students: you may assume that you are using a large excess of benzene as your starting material. C T Add/Remove step X ноarrow_forwardWhich one of the following atoms should have the largest electron affinity? a) b) c) d) 으으 e) 1s² 2s² 2p6 3s¹ 1s² 2s² 2p5 1s² 2s² 2p 3s² 3p² 1s² 2s 2p 3s² 3p6 4s2 3ds 1s² 2s² 2p6arrow_forward
- All of the following are allowed energy levels except _. a) 3f b) 1s c) 3d d) 5p e) 6sarrow_forwardA student wants to make the following product in good yield from a single transformation step, starting from benzene. Add any organic reagents the student is missing on the left-hand side of the arrow, and any addition reagents that are necessary above or below the arrow. If this product can't be made in good yield with a single transformation step, check the box below the drawing area. Note for advanced students: you may assume that an excess of benzene is used as part of the reaction conditions. : ☐ + I X This product can't be made in a single transformation step.arrow_forwardPredict the major products of this organic reaction:arrow_forward
- Name the family to which each organic compound belongs. The first answer has been filled in for you. compound CH₂ || CH3-C-NH2 0 ။ CH3-C-CH₂ CH=O–CH=CH, CH₂ HO CH2-CH2-CH-CH3 family amine Darrow_forward1b. Br LOHarrow_forwardI would like my graphs checked please. Do they look right? Do I have iodine and persulfate on the right axis ?arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


