
Concept explainers
a)
Interpretation:
The configurational stereochemistry of the molecules to be determined.
Answer to Problem 26VC
The Fischer projection follows as
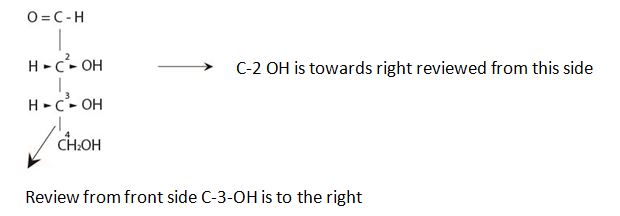
It is D sugar the molecule is a retrose. Elclose or eldotetrose, is 4-carbon elclose.
Explanation of Solution
Concept strategy: The Fischer projection of the given monosaccharide is drawn vertically, by rotating the molecule anticlockwise 90° so that the carbonyl
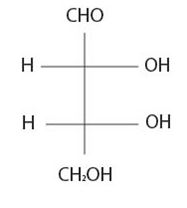
Review from front side C-3-OH is to the right By convention the molecule has the C-3 hyduxyl at the right. So it is D sugar the molecule is a retrose. Elclose or eldotetrose, is 4-carbon elclose.
Based on the Fischer projection formula for the given sugars it is a B-D-glucopyranose monosaccharide.
b)
Interpretation:
The configurational stereochemistry of the molecules to be determined.
Answer to Problem 26VC
The given model is the cyclic structures of an aldohexose in six membered pyranose form.
Strategy: We redraw the model as
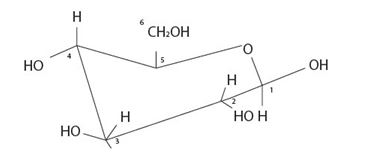
Explanation of Solution
By convention, the terminal -CH2OH group is on the top of the chair Pyranose structure. Thus it is a D sugar. The molecule is an aldohexose is B-D-glucopyranose, all the –OH groups are equatorial (and more stable due to minimum repulsion) conformation.
Based on the Fischer projection formula for the given sugars it is a B-D-glucopyranose monosaccharide.
Want to see more full solutions like this?
Chapter 25 Solutions
OWLv2 with Student Solutions Manual eBook, 4 terms (24 months) Printed Access Card for McMurry's Organic Chemistry, 9th
- A covalent bond is the result of the a) b) c) d) e) overlap of two half-filled s orbitals overlap of a half-filled s orbital and a half-filled p orbital overlap of two half-filled p orbitals along their axes parallel overlap of two half-filled parallel p orbitals all of the abovearrow_forwardCan the target compound at right be efficiently synthesized in good yield from the unsubstituted benzene at left? starting material target If so, draw a synthesis below. If no synthesis using reagents ALEKS recognizes is possible, check the box under the drawing area. Be sure you follow the standard ALEKS rules for submitting syntheses. + More... Note for advanced students: you may assume that you are using a large excess of benzene as your starting material. C T Add/Remove step X ноarrow_forwardWhich one of the following atoms should have the largest electron affinity? a) b) c) d) 으으 e) 1s² 2s² 2p6 3s¹ 1s² 2s² 2p5 1s² 2s² 2p 3s² 3p² 1s² 2s 2p 3s² 3p6 4s2 3ds 1s² 2s² 2p6arrow_forward
- All of the following are allowed energy levels except _. a) 3f b) 1s c) 3d d) 5p e) 6sarrow_forwardA student wants to make the following product in good yield from a single transformation step, starting from benzene. Add any organic reagents the student is missing on the left-hand side of the arrow, and any addition reagents that are necessary above or below the arrow. If this product can't be made in good yield with a single transformation step, check the box below the drawing area. Note for advanced students: you may assume that an excess of benzene is used as part of the reaction conditions. : ☐ + I X This product can't be made in a single transformation step.arrow_forwardPredict the major products of this organic reaction:arrow_forward
- Name the family to which each organic compound belongs. The first answer has been filled in for you. compound CH₂ || CH3-C-NH2 0 ။ CH3-C-CH₂ CH=O–CH=CH, CH₂ HO CH2-CH2-CH-CH3 family amine Darrow_forward1b. Br LOHarrow_forwardI would like my graphs checked please. Do they look right? Do I have iodine and persulfate on the right axis ?arrow_forward
