
Principles of Instrumental Analysis
7th Edition
ISBN: 9781305577213
Author: Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 19, Problem 19.33QAP
Interpretation Introduction
Interpretation:
The name of the organic compound from the following proton NMR data should be predicted.
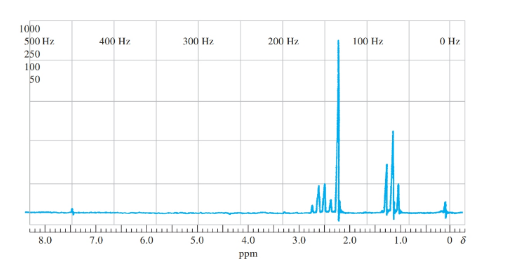
Concept introduction:
Nuclear magnetic resonance is a technique that is used to predict the structural formula of the compound. NMR spectroscopy involves the examination of the nucleus under the external magnetic field.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
achieve.macmillanlearning.com
Canvas
EA eac
h Hulu
YouTube
G 3 methyl cyclobutanol - Google Search
Ranking Phenol Acidity
Course -236 - Organic Chemistry - Mac...
←
Assessment
Completed 10 of 22 Questions
1 +
Netflix
paramount plus
chem hw
Galdehyde reaction with grignard reagent...
b My Questions | bartleby
M Inbox - chenteislegit@gmail.com - Gmail
Due: Fri, Jan 31
Resources
Solution
Penalized
? Hint
Submit Answer
Use retrosynthetic analysis to suggest two paths to synthesize 2-methyl-3-hexanol using the Grignard reaction. (Click and drag
the appropriate image to the correct position in the reactions.)
Route 1
Aldehyde 1
or
+98
Aldehyde 2
Route 2
Q6
+100
Solved in 1 attempt
Q7
+95
Solved in 2 attempts
Q8
+98
Unlimited attempts
possible
+
+
Grignard 1
OH
H3O+
Grignard 2
Answer Bank
Q9
+90
MgBr
Unlimited attempts
possible
CH3CH2CH2MgBr
Q10
Unlimited attempts
Q11
?
?
+100
in 1 attempt
2-methyl-3-hexanol
CH3CH2MgBr
H
H
о
H
Attempt 3
2) (4 pt) After the reaction was completed, the student collected the following data. Crude
product data is the data collected after the reaction is finished, but before the product
is purified. "Pure" product data is the data collected after attempted purification using
recrystallization.
Student B's data:
Crude product data
"Pure"
product data
after
recrystallization
Crude mass: 0.93 g grey solid
Crude mp: 96-106 °C
Crude % yield:
Pure mass: 0.39 g white solid
Pure mp: 111-113 °C
Pure % yield:
a) Calculate the crude and pure percent yields for the student's reaction.
b) Summarize what is indicated by the crude and pure melting points.
Don't used hand raiting
Chapter 19 Solutions
Principles of Instrumental Analysis
Ch. 19 - Prob. 19.1QAPCh. 19 - Prob. 19.2QAPCh. 19 - Prob. 19.3QAPCh. 19 - Prob. 19.4QAPCh. 19 - Prob. 19.5QAPCh. 19 - A nucleus has a spin quantum number of 7/2. How...Ch. 19 - Prob. 19.7QAPCh. 19 - Prob. 19.8QAPCh. 19 - Prob. 19.9QAPCh. 19 - Why is 133C-133C spin-spin splitting not observed...
Ch. 19 - Prob. 19.11QAPCh. 19 - Prob. 19.12QAPCh. 19 - Prob. 19.13QAPCh. 19 - What is a rotating frame of reference?Ch. 19 - How will E for an isolated 13C nucleus compare...Ch. 19 - Prob. 19.16QAPCh. 19 - Prob. 19.17QAPCh. 19 - Prob. 19.18QAPCh. 19 - Prob. 19.19QAPCh. 19 - Prob. 19.20QAPCh. 19 - Prob. 19.21QAPCh. 19 - Prob. 19.22QAPCh. 19 - Prob. 19.23QAPCh. 19 - Prob. 19.24QAPCh. 19 - Prob. 19.25QAPCh. 19 - Prob. 19.26QAPCh. 19 - Prob. 19.27QAPCh. 19 - Prob. 19.28QAPCh. 19 - Prob. 19.29QAPCh. 19 - Prob. 19.30QAPCh. 19 - The proton NMR spectrum in Figure 19.39 is for an...Ch. 19 - The proton NMR spectrum in Figure 19-40 is for a...Ch. 19 - Prob. 19.33QAPCh. 19 - Prob. 19.34QAPCh. 19 - Prob. 19.35QAPCh. 19 - From the proton NMR spectrum in Figure 19-44,...Ch. 19 - From the proton spectrum given in Figure 19-45,...Ch. 19 - Prob. 19.38QAPCh. 19 - Prob. 19.39QAPCh. 19 - Prob. 19.40QAPCh. 19 - Prob. 19.41QAPCh. 19 - Prob. 19.42QAP
Knowledge Booster
Similar questions
- A DEPT NMR spectrum is shown for a molecule with the molecular formula of C5H12O. Draw the structure that best fits this data. 200 180 160 140 120 100 一盆 00 40 8- 20 ppm 0 Qarrow_forwardDon't used hand raitingarrow_forwardShown below is the major resonance structure for a molecule. Draw the second best resonance structure of the molecule. Include all non-zero formal charges. H. H. +N=C H H H Cl: Click and drag to start drawing a structure. : ? g B S olo Ar B Karrow_forward
- Don't used hand raitingarrow_forwardS Shown below is the major resonance structure for a molecule. Draw the second best resonance structure of the molecule. Include all non-zero formal charges. H H = HIN: H C. :0 H /\ H H Click and drag to start drawing a structure. ×arrow_forwardPlease help me figure out these calculation and what should be plotted. These are notes for my chemistry class.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,