
Principles of Instrumental Analysis
7th Edition
ISBN: 9781305577213
Author: Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 19, Problem 19.37QAP
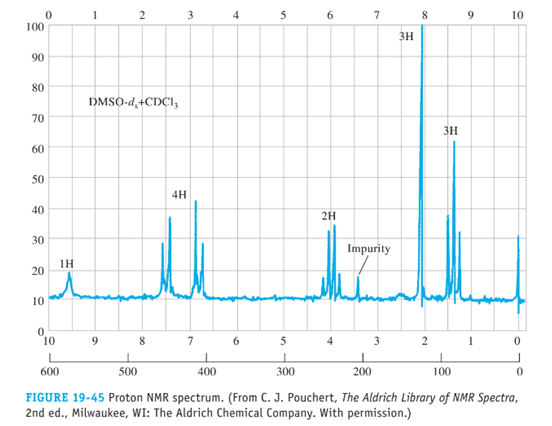
From the proton spectrum given in Figure 19-45, determine the structure of this compound, a commc used painkiller; its empirical formula is C10H13NO2.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
•
Part II.
a) Elucidate The structure of compound c w/ molecular formula C10 11202 and the following data below:
• IR spectra
% TRANSMITTANCE
1002.5
90
80
70
60
50
40
30
20
10
0
4000
3600
3200
2800
2400
2000
1800
1600
• Information from 'HAMR
MICRONS
8 9 10
11
14 15 16
19
25
1400
WAVENUMBERS (CM-1)
1200
1000
800
600
400
peak
8 ppm
Integration
multiplicity
a
2.1
1.5 (3H)
Singlet
b
3.6
1 (2H)
singlet
с
3.8
1.5 (3H)
Singlet
d
6.8
1(2H)
doublet
7.1
1(2H)
doublet
Information from 13C-nmR
Normal carbon
29ppm
Dept 135
Dept -90
+
NO peak
NO peak
50 ppm
55 ppm
+
NO peak
114 ppm
t
126 ppm
No peak
NO peak
130 ppm
t
+
159 ppm
No peak
NO peak
207 ppm
по реак
NO peak
Could you redraw these and also explain how to solve them for me pleas
None
Chapter 19 Solutions
Principles of Instrumental Analysis
Ch. 19 - Prob. 19.1QAPCh. 19 - Prob. 19.2QAPCh. 19 - Prob. 19.3QAPCh. 19 - Prob. 19.4QAPCh. 19 - Prob. 19.5QAPCh. 19 - A nucleus has a spin quantum number of 7/2. How...Ch. 19 - Prob. 19.7QAPCh. 19 - Prob. 19.8QAPCh. 19 - Prob. 19.9QAPCh. 19 - Why is 133C-133C spin-spin splitting not observed...
Ch. 19 - Prob. 19.11QAPCh. 19 - Prob. 19.12QAPCh. 19 - Prob. 19.13QAPCh. 19 - What is a rotating frame of reference?Ch. 19 - How will E for an isolated 13C nucleus compare...Ch. 19 - Prob. 19.16QAPCh. 19 - Prob. 19.17QAPCh. 19 - Prob. 19.18QAPCh. 19 - Prob. 19.19QAPCh. 19 - Prob. 19.20QAPCh. 19 - Prob. 19.21QAPCh. 19 - Prob. 19.22QAPCh. 19 - Prob. 19.23QAPCh. 19 - Prob. 19.24QAPCh. 19 - Prob. 19.25QAPCh. 19 - Prob. 19.26QAPCh. 19 - Prob. 19.27QAPCh. 19 - Prob. 19.28QAPCh. 19 - Prob. 19.29QAPCh. 19 - Prob. 19.30QAPCh. 19 - The proton NMR spectrum in Figure 19.39 is for an...Ch. 19 - The proton NMR spectrum in Figure 19-40 is for a...Ch. 19 - Prob. 19.33QAPCh. 19 - Prob. 19.34QAPCh. 19 - Prob. 19.35QAPCh. 19 - From the proton NMR spectrum in Figure 19-44,...Ch. 19 - From the proton spectrum given in Figure 19-45,...Ch. 19 - Prob. 19.38QAPCh. 19 - Prob. 19.39QAPCh. 19 - Prob. 19.40QAPCh. 19 - Prob. 19.41QAPCh. 19 - Prob. 19.42QAP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the curved-arrow mechanism with the drawings of the molecules, not just abbreviations. -NO₂ Sn, HCl (aq) E D H (CH3CO)₂O -NH2 CH3arrow_forwardWhat is/are the product(s) of the following reaction? Select all that apply. * HI A B C OD OH A B OH D Carrow_forwardIn the image, the light blue sphere represents a mole of hydrogen atoms, the purple or teal spheres represent a mole of a conjugate base. A light blue sphere by itself is H+. Assuming there is 2.00 L of solution, answer the following: The Ka of the left & right solution is? The pH of the left & right solution is? The acid on the left & right is what kind of acid?arrow_forward
- What spectral features allow you to differentiate the product from the starting material? Use four separate paragraphs for each set of comparisons. You should have one paragraph each devoted to MS, HNMR, CNMR and IR. 2) For MS, the differing masses of molecular ions are a popular starting point. Including a unique fragmentation is important, too. 3) For HNMR, CNMR and IR state the peaks that are different and what makes them different (usually the presence or absence of certain groups). See if you can find two differences (in each set of IR, HNMR and CNMR spectra) due to the presence or absence of a functional group. Include peak locations. Alternatively, you can state a shift of a peak due to a change near a given functional group. Including peak locations for shifted peaks, as well as what these peaks are due to. Ideally, your focus should be on not just identifying the differences but explaining them in terms of functional group changes.arrow_forwardQuestion 6 What is the major product of the following Diels-Alder reaction? ? Aldy by day of A. H о B. C. D. E. OB OD Oc OE OAarrow_forwardNonearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY