
Principles of Instrumental Analysis
7th Edition
ISBN: 9781305577213
Author: Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 19, Problem 19.36QAP
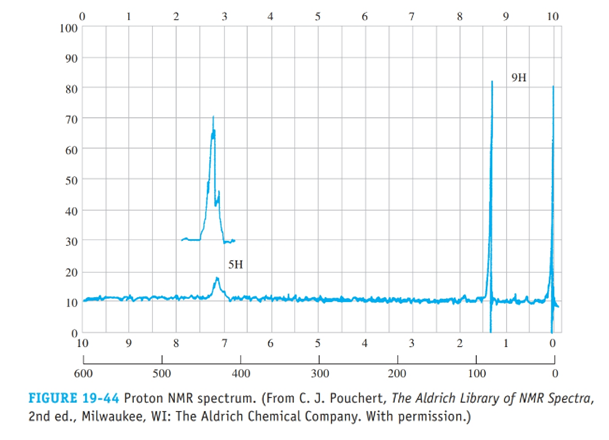
From the proton NMR spectrum in Figure 19-44, deduce the structure of this hydrocarbon
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
The quantum yield of the photochemical decay of HI is 2. Calculate the number of Einsteins absorbed per mole knowing that the energy absorbed per mole of photons is 490 kJ.
The quantum yield of the photochemical decay of HI is 2. How many moles of HI per kJ of radiant energy can be decayed knowing that the energy absorbed per mole of photons is 490 kJ.
If the energy absorbed per mole of photons is 450 kJ, the number of Einsteins absorbed per 1 mole.
Chapter 19 Solutions
Principles of Instrumental Analysis
Ch. 19 - Prob. 19.1QAPCh. 19 - Prob. 19.2QAPCh. 19 - Prob. 19.3QAPCh. 19 - Prob. 19.4QAPCh. 19 - Prob. 19.5QAPCh. 19 - A nucleus has a spin quantum number of 7/2. How...Ch. 19 - Prob. 19.7QAPCh. 19 - Prob. 19.8QAPCh. 19 - Prob. 19.9QAPCh. 19 - Why is 133C-133C spin-spin splitting not observed...
Ch. 19 - Prob. 19.11QAPCh. 19 - Prob. 19.12QAPCh. 19 - Prob. 19.13QAPCh. 19 - What is a rotating frame of reference?Ch. 19 - How will E for an isolated 13C nucleus compare...Ch. 19 - Prob. 19.16QAPCh. 19 - Prob. 19.17QAPCh. 19 - Prob. 19.18QAPCh. 19 - Prob. 19.19QAPCh. 19 - Prob. 19.20QAPCh. 19 - Prob. 19.21QAPCh. 19 - Prob. 19.22QAPCh. 19 - Prob. 19.23QAPCh. 19 - Prob. 19.24QAPCh. 19 - Prob. 19.25QAPCh. 19 - Prob. 19.26QAPCh. 19 - Prob. 19.27QAPCh. 19 - Prob. 19.28QAPCh. 19 - Prob. 19.29QAPCh. 19 - Prob. 19.30QAPCh. 19 - The proton NMR spectrum in Figure 19.39 is for an...Ch. 19 - The proton NMR spectrum in Figure 19-40 is for a...Ch. 19 - Prob. 19.33QAPCh. 19 - Prob. 19.34QAPCh. 19 - Prob. 19.35QAPCh. 19 - From the proton NMR spectrum in Figure 19-44,...Ch. 19 - From the proton spectrum given in Figure 19-45,...Ch. 19 - Prob. 19.38QAPCh. 19 - Prob. 19.39QAPCh. 19 - Prob. 19.40QAPCh. 19 - Prob. 19.41QAPCh. 19 - Prob. 19.42QAP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- When propionic aldehyde in vapor form at 200 mmHg and 30°C is irradiated with radiation of wavelength 302 nm, the quantum yield with respect to the formation of CO is 0.54. If the intensity of the incident radiation is 1.5x10-3 W, find the rate of formation of CO.arrow_forwardDraw mechanismarrow_forwardDoes Avogadro's number have units?arrow_forward
- Explain why the total E in an Einstein depends on the frequency or wavelength of the light.arrow_forwardIf the dissociation energy of one mole of O2 is 5.17 eV, determine the wavelength that must be used to dissociate it with electromagnetic radiation. Indicate how many Einstein's of this radiation are needed to dissociate 1 liter of O2 at 25°C and 1 atm of pressure.Data: 1 eV = 96485 kJ mol-1; R = 0.082 atm L K-1; c = 2.998x108 m s-1; h = 6.626x10-34 J s; NA = 6.022x 1023 mol-1arrow_forwardIndicate the number of Einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy (wavelength 475 nm).arrow_forward
- Indicate the number of einsteins that are equivalent to 550 kJ mol⁻¹ of absorbed energy?arrow_forwardA unit used in photochemistry is the einstein. If 400 kJ mol-1 of energy has been absorbed, how many einsteins is this equivalent to?arrow_forwardFor the condensation reaction between Alanine and histidine write the amididation reaction mechanism using arrows then write the three letter code for the product of the reaction and the one letter code for the product of the reaction.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY