
ORGANIC CHEMISTRY-PACKAGE >CUSTOM<
10th Edition
ISBN: 9781260028355
Author: Carey
Publisher: MCG CUSTOM
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 18, Problem 37P
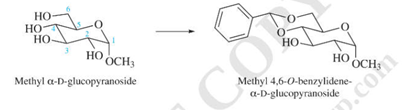
The OH groups at C-4 and C-6 of methyl α−
D-glucopyranoside can be protected by conversion to a benzylidene acetal. What reagents are needed for this conversion?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
K
←
nationa
Login - Paymentivet
MapQue
Draw the major product of this reaction. Ignore inorganic
byproducts and the carboxylic acid side product.
N
1. excess LiAlH4
2. H₂O
✓
W
a
Draw the major product of this reaction. Ignore inorganic
byproducts and the alcohol side product.
1. H3O+, heat
2. Neutralizing work-up
Drawing
Q
Indicate the procedure (reagent Z) to go from compound A1 to
compound A2.
A1
Z
P(C6H5)3
A2
Chapter 18 Solutions
ORGANIC CHEMISTRY-PACKAGE >CUSTOM<
Ch. 18.1 - Prob. 1PCh. 18.1 - Prob. 2PCh. 18.3 - Prob. 3PCh. 18.4 - Prob. 4PCh. 18.4 - Prob. 5PCh. 18.6 - Prob. 6PCh. 18.7 - Prob. 7PCh. 18.7 - Prob. 8PCh. 18.7 - Prob. 9PCh. 18.8 - Prob. 10P
Ch. 18.8 - Prob. 11PCh. 18.8 - Prob. 12PCh. 18.9 - Prob. 13PCh. 18.10 - Prob. 14PCh. 18.10 - Prob. 15PCh. 18.11 - Problem 18.16 The product of the following...Ch. 18.11 - Prob. 17PCh. 18.12 - Problem 18.18 What other combination of ylide and...Ch. 18.12 - Prob. 19PCh. 18.12 - Prob. 20PCh. 18.12 - Prob. 21PCh. 18.13 - Prob. 22PCh. 18 - (a) Write structural formulas and provide IUPAC...Ch. 18 - Each of the following aldehydes and ketones is...Ch. 18 - The African dwarf crocodile secretes a volatile...Ch. 18 - Prob. 26PCh. 18 - Prob. 27PCh. 18 - Prob. 28PCh. 18 - Prob. 29PCh. 18 - Prob. 30PCh. 18 - Prob. 31PCh. 18 - Each of the following reaction has been reported...Ch. 18 - Prob. 33PCh. 18 - On standing in 17O-labeled water, both...Ch. 18 - Prob. 35PCh. 18 - Prob. 36PCh. 18 - The OH groups at C-4 and C-6 of methyl ...Ch. 18 - Prob. 38PCh. 18 - Prob. 39PCh. 18 - The sex attractant of the female winter moth has...Ch. 18 - Prob. 41PCh. 18 - Prob. 42PCh. 18 - Prob. 43PCh. 18 - Suggest a reasonable mechanism for each of the...Ch. 18 - Prob. 45PCh. 18 - Prob. 46PCh. 18 - Prob. 47PCh. 18 - Prob. 48PCh. 18 - Prob. 49PCh. 18 - Prob. 50PCh. 18 - Prob. 51PCh. 18 - Prob. 52DSPCh. 18 - Prob. 53DSPCh. 18 - Prob. 54DSPCh. 18 - Prob. 55DSPCh. 18 - Prob. 56DSPCh. 18 - Prob. 57DSPCh. 18 - Prob. 58DSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please help with this graph.arrow_forwardogin - PaymentN MapQuest 3 Overview - SAP NetW... Draw the major product of this reaction. Ignore inorganic byproducts. CI 1. NaBH4 2. H₂O C Clever | Portal Job Op Problem Atoms, Bonds and Rings Draw or tap a new bond toarrow_forward2. Draw the remaining two resonance structures for the carbocation intermediate in the meta nitration of methyl benzoate AND explain why the meta orientation is preferred. Hint: how is the placement of the cation favorable after addition of nitronium relative to the electron withdrawing group? (2 pts) H NO2 CO₂Mearrow_forward
- Label all absorptions over 1500 cm-1. Please be specific and mark IR if needed for explanation. Compound OH sp^3 C-H C=O C-O Triglyceridearrow_forwardIdentify the intermediate that is INITIALLY formed in a saponification reaction (hydrolysis of an ester). III -OH H₂O HO OH HO O || A B C III D IV IVarrow_forwardHelp me answer this practice sheet I found for an answer guidearrow_forward
- show the retrosynthesis of this molecule step by step starting with 1,3-dimethoxy benzene H3CO OH OH OCH 3arrow_forwardConsider the reaction of a propanoate ester with hydroxide ion shown below. A series of four alcohol leaving groups were tested to determine which would be the best leaving group. Based on the pKa values of the alcohols, predict which alcohol would produce the fastest hydrolysis reaction. HO FOR A Alcohol I, pKa =16.0 B Alcohol II, pKa =10.0 C Alcohol III, pKa = 7.2 + ROH D Alcohol IV, pKa = 6.6arrow_forwardCurved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. :0: NaOH, H₂O 00:4 Na O heat NaO Select to Add Arrows Select to Add Arrows :0: Na a NaOH, H2O :0: NaOH, H2O heat heat Na ONH Select to Add Arrowsarrow_forward
- Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. H CH3NH3+ :0: :0: HO CH3NH2 HH iSelect to Add Arrows i Select to Add Arrows i HH CH3NH3+ CH3NH2 Select to Add Arrows i CH3NH3 CH3NH2 ايكدا HH Select to Add Arrowsarrow_forwardThe reaction is carried out with gases: A → B + C at 300 K. The total pressure is measured as a function of time (table). If the reaction order is 2, calculate the rate or kinetic constant k (in mol-1 L s¹) Ptotal (atm) 492 676 760 808 861 t(s) 0 600 1200 1800 3000arrow_forwardcan someone give a description of this NMR including whether its a triplt singlet doublet where the peak is around at ppm and what functional group it representsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY