
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 18, Problem 29P
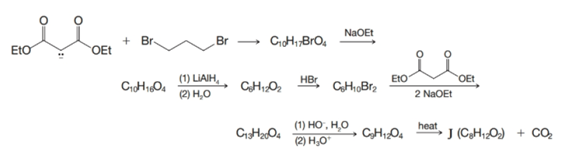
Compound J, a compound with two four-membered rings, has been synthesized by the following route. Outline the steps that are involved.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
When talking about the acidity of carboxylic acids, is it the same thing to say higher or stronger acidity?
Using the following two half-reactions, determine the pH range in which $NO_2^-\ (aq)$ cannot be found as the predominant chemical species in water.* $NO_3^-(aq)+10H^+(aq)+8e^-\rightarrow NH_4^+(aq)+3H_2O(l),\ pE^{\circ}=14.88$* $NO_2^-(aq)+8H^+(aq)+6e^-\rightarrow NH_4^+(aq)+2H_2O(l),\ pE^{\circ}=15.08$
Indicate characteristics of oxodec acid.
Chapter 18 Solutions
Organic Chemistry
Ch. 18 - Prob. 1PPCh. 18 - Practice Problem 18.2 Would optically active...Ch. 18 - Prob. 3PPCh. 18 - Practice Problem 18.4 Why do we say that the...Ch. 18 - Prob. 5PPCh. 18 - Practice Problem 18.6 (a) Write a reaction...Ch. 18 - PRACTICE PROBLEM 18.7
Show how you would use the...Ch. 18 - Practice Problem 18.8 The acetoacetic ester...Ch. 18 - Practice Problem 18.9
In the synthesis of the keto...Ch. 18 - PRACTICE PROBLEM 18.10 How would you use the...
Ch. 18 - PRACTICE PROBLEM 18.11
How would you use the...Ch. 18 - PRACTICE PROBLEM 18.12 Outline all steps in a...Ch. 18 - PRACTICE PROBLEM 18.13
The antiepileptic drug...Ch. 18 - PRACTICE PROBLEM 18.14 Show how you could employ...Ch. 18 - Prob. 15PCh. 18 - Treating a solution of cis-1-decalone with base...Ch. 18 - Prob. 17PCh. 18 - Prob. 18PCh. 18 - Prob. 19PCh. 18 - Prob. 20PCh. 18 - Prob. 21PCh. 18 - Prob. 22PCh. 18 - Prob. 23PCh. 18 - The synthesis of cyclobutanecarboxylic acid given...Ch. 18 - Prob. 25PCh. 18 - Prob. 26PCh. 18 - Prob. 27PCh. 18 - Prob. 28PCh. 18 - Compound J, a compound with two four-membered...Ch. 18 - Prob. 30PCh. 18 - Prob. 31PCh. 18 - 18.32 Shown below is a synthesis of the elm bark...Ch. 18 - 18.33 (a) A compound U gives a negative iodoform...Ch. 18 - 18.34 Compound A has the molecular formula and...Ch. 18 - Prob. 35PCh. 18 - Prob. 36PCh. 18 - Prob. 37PCh. 18 - Prob. 38PCh. 18 - 1. -Carotene is a highly conjugated hydrocarbon...Ch. 18 - Dehydroabietic acid is a natural product isolated...
Additional Science Textbook Solutions
Find more solutions based on key concepts
In the overhead view of Fig. 9-54, a 300 g ball with a speed v of 6.0 m/s strikes a wall at an angle of 30 and...
Fundamentals of Physics Extended
17. How does the presence of a nonvolatile solute affect the boiling point and melting point of a solution rel...
Introductory Chemistry (6th Edition)
How can the freezing of water crack boulders?
Campbell Biology in Focus (2nd Edition)
Another cross in Drosophila involved the recessive, X-linked genes yellow (y), white (w), and cut (ct). A yello...
Concepts of Genetics (12th Edition)
The accompanying chromosome diagram represents a eukaryotic chromosome prepared with Giemsa stain. Indicate the...
Genetic Analysis: An Integrated Approach (3rd Edition)
11. Birds and mammals are both endothermic, and both have four-chambered hearts. Most reptiles are ectothermic ...
Campbell Biology: Concepts & Connections (9th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the final product when hexanedioic acid reacts with 1º PCl5 and 2º NH3.arrow_forwardWhat is the final product when D-galactose reacts with hydroxylamine?arrow_forwardIndicate the formula of the product obtained by reacting methyl 5-chloro-5-oxopentanoate with 1 mole of 4-penten-1-ylmagnesium bromide.arrow_forward
- The temperature on a sample of pure X held at 1.25 atm and -54. °C is increased until the sample boils. The temperature is then held constant and the pressure is decreased by 0.42 atm. On the phase diagram below draw a path that shows this set of changes. pressure (atm) 2 0 0 200 400 temperature (K) Xarrow_forwardQUESTION: Answer Question 5: 'Calculating standard error of regression' STEP 1 by filling in all the empty green boxes *The values are all provided in the photo attached*arrow_forwardpressure (atm) 3 The pressure on a sample of pure X held at 47. °C and 0.88 atm is increased until the sample condenses. The pressure is then held constant and the temperature is decreased by 82. °C. On the phase diagram below draw a path that shows this set of changes. 0 0 200 temperature (K) 400 аarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

Characteristic Reactions of Benzene and Phenols; Author: Linda Hanson;https://www.youtube.com/watch?v=tjEqEjDd87E;License: Standard YouTube License, CC-BY
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=-fBPX-4kFlw;License: Standard Youtube License