
Organic Chemistry
12th Edition
ISBN: 9781118875766
Author: T. W. Graham Solomons, Craig B. Fryhle, Scott A. Snyder
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 18, Problem 16P
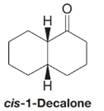
Treating a solution of cis-1-decalone with base causes an isomerization to take place. When the system reaches equilibrium, the solution is found to contain about 95% trans-1-decalone and about 5% cis-1-decalone. Explain this isomerization.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Select the product for the following reaction.
HO
HO
PCC
OH
○
OH
O HO
○ HO
HO
HO
5:45
Х
Select the final product for the following reaction sequence.
O
O
1. Mg. ether
2.D.O
Based on the chart
Two similarities between the molecule with alpha glycosidic linkages.
Two similarities between the molecules with beta glycosidtic linkages.
Two differences between the alpha and beta glycosidic linkages.
Chapter 18 Solutions
Organic Chemistry
Ch. 18 - Prob. 1PPCh. 18 - Practice Problem 18.2 Would optically active...Ch. 18 - Prob. 3PPCh. 18 - Practice Problem 18.4 Why do we say that the...Ch. 18 - Prob. 5PPCh. 18 - Practice Problem 18.6 (a) Write a reaction...Ch. 18 - PRACTICE PROBLEM 18.7
Show how you would use the...Ch. 18 - Practice Problem 18.8 The acetoacetic ester...Ch. 18 - Practice Problem 18.9
In the synthesis of the keto...Ch. 18 - PRACTICE PROBLEM 18.10 How would you use the...
Ch. 18 - PRACTICE PROBLEM 18.11
How would you use the...Ch. 18 - PRACTICE PROBLEM 18.12 Outline all steps in a...Ch. 18 - PRACTICE PROBLEM 18.13
The antiepileptic drug...Ch. 18 - PRACTICE PROBLEM 18.14 Show how you could employ...Ch. 18 - Prob. 15PCh. 18 - Treating a solution of cis-1-decalone with base...Ch. 18 - Prob. 17PCh. 18 - Prob. 18PCh. 18 - Prob. 19PCh. 18 - Prob. 20PCh. 18 - Prob. 21PCh. 18 - Prob. 22PCh. 18 - Prob. 23PCh. 18 - The synthesis of cyclobutanecarboxylic acid given...Ch. 18 - Prob. 25PCh. 18 - Prob. 26PCh. 18 - Prob. 27PCh. 18 - Prob. 28PCh. 18 - Compound J, a compound with two four-membered...Ch. 18 - Prob. 30PCh. 18 - Prob. 31PCh. 18 - 18.32 Shown below is a synthesis of the elm bark...Ch. 18 - 18.33 (a) A compound U gives a negative iodoform...Ch. 18 - 18.34 Compound A has the molecular formula and...Ch. 18 - Prob. 35PCh. 18 - Prob. 36PCh. 18 - Prob. 37PCh. 18 - Prob. 38PCh. 18 - 1. -Carotene is a highly conjugated hydrocarbon...Ch. 18 - Dehydroabietic acid is a natural product isolated...
Additional Science Textbook Solutions
Find more solutions based on key concepts
3. What is free-fall, and why does it make you weightless? Briefly describe why astronauts are weightless in th...
The Cosmic Perspective (8th Edition)
2 Of the uterus, small intestine, spinal cord, and heart, which is/are in the dorsal body cavity?
Anatomy & Physiology (6th Edition)
Give your own example of how the structure of a part of the body is related to its function.
Principles of Anatomy and Physiology
A source of electromagnetic radiation produces infrared light. Which of the following could be the wavelength ...
Chemistry: The Central Science (14th Edition)
39. What are the units of k for each type of reaction?
a. first-order reaction
b. second-order reaction
c...
Chemistry: Structure and Properties (2nd Edition)
The magnetic component of an electromagnetic wave in vacuum has an amplitude of 85.8 nT and an angular wave num...
Fundamentals of Physics Extended
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- please help fill in the tablearrow_forwardAnswer F pleasearrow_forward4. Refer to the data below to answer the following questions: The octapeptide saralasin is a specific antagonist of angiotensin II. A derivative of saralasin is used therapeutically as an antihypertensive. Amino acid analysis of saralasin show the presence of the following amino acids: Ala, Arg, His, Pro, Sar, Tyr, Val, Val A.Sar is the abbreviation for sarcosine, N-methyl aminoethanoic acid. Draw the structure of sarcosine. B. N-Terminal analysis by the Edman method shows saralasin contains sarcosine at the N-terminus. Partial hydrolysis of saralasin with dilute hydrochloric acid yields the following fragments: Tyr-Val-His Sar-Arg-Val His-Pro-Ala Val-Tyr-Val Arg-Val-Tyr What is the structure of saralasin?arrow_forward
- What is the structure of the DNA backbone?arrow_forwardPLEASE PLEASE PLEASE use hand drawn structures when possarrow_forward. M 1- MATCH each of the following terms to a structure from the list below. There is only one correct structure for each term and structures may be used more than once. Place the letter of the structure in the blank to the left of the corresponding term. A. Sanger dideoxy method C. Watson-Crick B. GAUCGUAAA D. translation E. HOH2C OH OH G. transcription I. AUGGCUGAG 0 K. OPOH2C 0- OH N- H NH2 F. -OPOH2C 0- OH OH H. Maxam-Gilbert method J. replication N L. HOH2C a. b. C. d. e. f. g. B M. AGATCGCTC a pyrimidine nucleoside RNA base sequence with guanine at the 3' end. DNA base sequence with cytosine at the 3' end. a purine nucleoside DNA sequencing method for the human genome 2'-deoxyadenosine 5'-phosphate process by which mRNA directs protein synthesis OH NH2arrow_forward
- Please use hand drawn structures when neededarrow_forwardB. Classify the following amino acid. Atoms other than carbon and hydrogen are labeled. a. acidic b. basic C. neutral C. Consider the following image. Which level of protein structure is shown here? a. primary b. secondary c. tertiary d. quaternary D. Consider the following image. H RH H HR H R HR HR RH Which level of protein structure is shown in the box? a. primary b. secondary R c. tertiary d. quaternary コー Rarrow_forwardBriefly answer three from the followings: a. What are the four structures of the protein? b. Why is the side chain (R) attached to the alpha carbon in the amino acids is important for the function? c. What are the types of amino acids? And how is it depend on the (R) structure? d. Write a reaction to prepare an amino acid. prodarrow_forward
- Answe Answer A and B pleasearrow_forward3. Refer to the data below to answer the following questions: Isoelectric point Amino Acid Arginine 10.76 Glutamic Acid 3.22 Tryptophan 5.89 A. Define isoelectric point. B. The most basic amino acid is C. The most acidic amino acid is sidizo zoarrow_forward3. A gas mixture contains 50 mol% H2 and 50 mol% He. 1.00-L samples of this gas mixture are mixed with variable volumes of O2 (at 0 °C and 1 atm). A spark is introduced to allow the mixture to undergo complete combustion. The final volume is measured at 0 °C and 1 atm. Which graph best depicts the final volume as a function of the volume of added O2? (A) 2.00 1.75 Final Volume, L 1.50 1.25 1.00 0.75 0.50 0.25 0.00 0.00 0.25 0.50 2.00 (B) 1.75 1.50 Final Volume, L 1.25 1.00 0.75 0.50- 0.25 0.00 0.75 1.00 0.00 0.25 Volume O₂ added, L 2 0.50 0.75 1.00 Volume O₂ added, L 2 2.00 2.00 (C) (D) 1.75 1.75 1.50 1.50 Final Volume, L 1.25 1.00 0.75 0.50 Final Volume, L 1.25 1.00 0.75 0.50 0.25 0.25 0.00 0.00 0.00 0.25 0.50 0.75 1.00 0.00 0.25 Volume O₂ added, L 0.50 0.75 1.00 Volume O₂ added, L 2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY