
Concept explainers
(a)
Interpretation:
Whether the fatty acid
Concept introduction:
A fatty acid having a long aliphatic chain with no double bond is called saturated fatty acid and a fatty acid that contains at least one double bond within the fatty acid chain is called unsaturated fatty acid. Due to the presence of a long alkyl side chain in saturated fatty acids, their packing efficiency is more than those of unsaturated fatty acids. As a result, unsaturated fatty acids possess lower melting point than saturated fatty acids. Thus, most of the saturated fatty acids are solid at room temperature.
Answer to Problem 18.9E
The fatty acid
Explanation of Solution
The structural formula of fatty acid
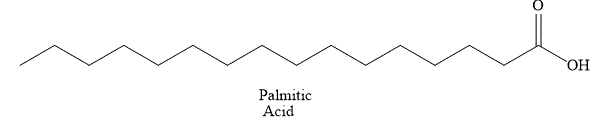
Figure 1
The general name of the above fatty acid is palmitic acid. Palmitic acid has a long aliphatic chain of
The fatty acid shown in Figure 1 is a saturated fatty acid and it is solid at room temperature.
(b)
Interpretation:
Whether the fatty acid
Concept introduction:
A fatty acid having a long aliphatic chain with no double bond is called saturated fatty acid and a fatty acid that contains at least one double bond within the fatty acid chain is called unsaturated fatty acid. Due to the presence of a long alkyl side chain in saturated fatty acids, their packing efficiency is more than those of unsaturated fatty acids. As a result, unsaturated fatty acids possess lower melting point than saturated fatty acids. Thus, most of the saturated fatty acids are solid at room temperature.
Answer to Problem 18.9E
The fatty acid
Explanation of Solution
The structural formula of fatty acid

Figure 2
The general name of the above fatty acid is lenoleic acid. The structural formula of lenoleic acid shows that there are two double bonds in the carbon chain. Therefore, lenoleic acid is an unsaturated fatty acid. The side chain in unsaturated fatty acids possess less van der Waal’s interactions between side chains of adjacent molecule. Therefore, the unsaturated fatty acid is a liquid at room temperature.
The fatty acid shown in Figure 2 is an unsaturated fatty acid and it is a liquid at room temperature.
(c)
Interpretation:
Whether the fatty acid
Concept introduction:
A fatty acid having a long aliphatic chain with no double bond is called saturated fatty acid and a fatty acid that contains at least one double bond within the fatty acid chain is called unsaturated fatty acid. Due to the presence of a long alkyl side chain in saturated fatty acids, their packing efficiency is more than those of unsaturated fatty acids. As a result, unsaturated fatty acids possess lower melting point than saturated fatty acids. Thus, most of the saturated fatty acids are solid at room temperature.
Answer to Problem 18.9E
The fatty acid
Explanation of Solution
The structural formula of fatty acid

Figure 3
The general name of the above fatty acid is palmitolic acid. The structural formula of palmitolic acid contains double bonds within the fatty acid chain. Therefore, palmitolic acid is an unsaturated fatty acid. The side chain in unsaturated fatty acids possess less van der Waal’s interactions between side chains of adjacent molecule. Therefore, the unsaturated fatty acid is a liquid at room temperature.
The fatty acid shown in Figure 3 is an unsaturated fatty acid and it is a liquid at room temperature.
(d)
Interpretation:
Whether the fatty acid
Concept introduction:
A fatty acid having a long aliphatic chain with no double bond is called saturated fatty acid and a fatty acid that contains at least one double bond within the fatty acid chain is called unsaturated fatty acid. Due to the presence of a long alkyl side chain in saturated fatty acids, their packing efficiency is more than those of unsaturated fatty acids. As a result, unsaturated fatty acids possess lower melting point than saturated fatty acids. Thus, most of the saturated fatty acids are solid at room temperature.
Answer to Problem 18.9E
The fatty acid
Explanation of Solution
The structural formula of fatty acid

Figure 4
The general name of the above fatty acid is lauric acid. Lauric acid has a long aliphatic chain of
The fatty acid shown in Figure 4 is a saturated fatty acid and it is a solid at room temperature.
Want to see more full solutions like this?
Chapter 18 Solutions
Chemistry for Today: General, Organic, and Biochemistry
- I need help with the followingarrow_forwardFor Raman spectroscopy/imaging, which statement is not true regarding its disadvantages? a) Limited spatial resolution. b) Short integration time. c) A one-dimensional technique. d) Weak signal, only 1 in 108 incident photons is Raman scattered. e) Fluorescence interference.arrow_forwardUsing a cell of known pathlength b = 1.25115 x 10-3 cm, a water absorption spectrum was measured. The band at 1645 cm-1, assigned to the O-H bending, showed an absorbance, A, of 1.40. a) Assuming that water density is 1.00 g/mL, calculate the water molar concentration c (hint: M= mole/L) b) Calculate the molar absorptivity, a, of the 1645 cm-1 band c) The transmitted light, I, can be written as I= Ioexp(-xb), where x is the absorption coefficient (sometimes designated as alpha), Io is the input light, and b is the cell pathlength. Prove that x= (ln10)*x*c. (Please provide a full derivation of the equation for x from the equation for I). d) Calculate x for the 1645 cm-1 bandarrow_forward
- For CARS, which statement is not true regarding its advantages? a) Contrast signal based on vibrational characteristics, no need for fluorescent tagging. b) Stronger signals than spontaneous Raman. c) Suffers from fluorescence interference, because CARS signal is at high frequency. d) Faster, more efficient imaging for real-time analysis. e) Higher resolution than spontaneous Raman microscopy.arrow_forwardDraw the major product of the Claisen condensation reaction between two molecules of this ester. Ignore inorganic byproducts. Incorrect, 5 attempts remaining 1. NaOCH3/CH3OH 2. Acidic workup Select to Draw O Incorrect, 5 attempts remaining The total number of carbons in the parent chain is incorrect. Review the reaction conditions including starting materials and/or intermediate structures and recount the number of carbon atoms in the parent chain of your structure. OKarrow_forwardUsing a cell of known pathlength b = 1.25115 x 10-3 cm, a water absorption spectrum was measured. The band at 1645 cm-1, assigned to the O-H bending, showed an absorbance, A, of 1.40. a) Assuming that water density is 1.00 g/mL, calculate the water molar concentration c (hint: M= mole/L) b) Calculate the molar absorptivity, a, of the 1645 cm-1 band c) The transmitted light, I, can be written as I= Ioexp(-xb), where x is the absorption coefficient (sometimes designated as alpha), Io is the input light, and b is the cell pathlength. Prove that x= (ln10)*x*c d) Calculate x for the 1645 cm-1 bandarrow_forward
 Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





