
(a)
Interpretation:
The complete, detailed mechanism of a given reaction in the acidic medium is to be drawn and the major organic product is to be predicted.
Concept introduction:
When an
Answer to Problem 18.55P
The complete, detailed mechanism of the given reaction in the acidic medium is shown below and an acetal is the major product.
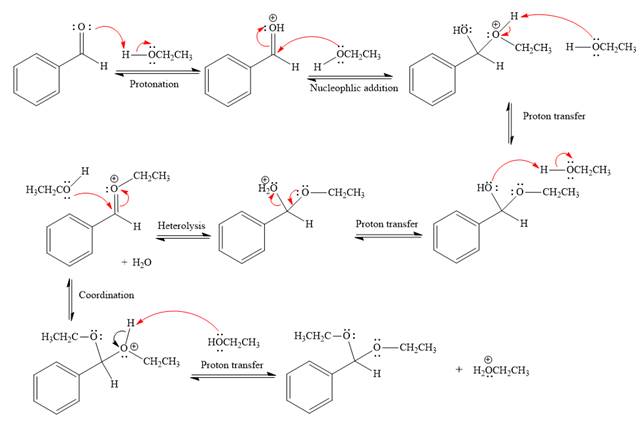
Explanation of Solution
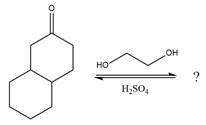
The given reaction is
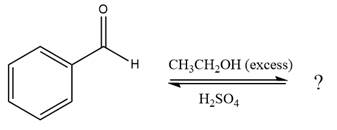
This is an acetal formation reaction in which the reaction is catalyzed by sulfuric acid and the excess ethanol acts as the nucleophile.
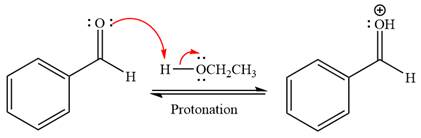
First three steps are acid catalyzed nucleophilic addition reactions on the ketone or aldehyde. In the first step, the
Next, the weak nucleophile, alcohol, attacks the activated electrophilic carbon by nucleophilic addition reaction.
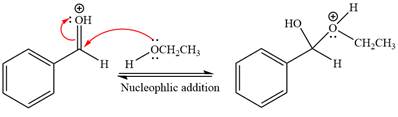
In the next step, deprotonation produces the uncharged hemiacetal.
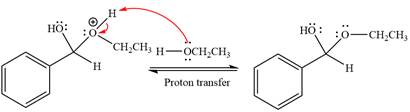
The remaining steps essentially make up
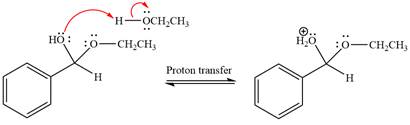
The
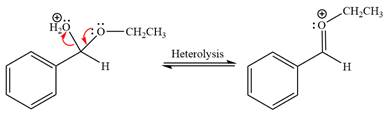
The resonance stabilized carbocation is further attacked by the ethyl alcohol nucleophile, which produces positively charged acetal.
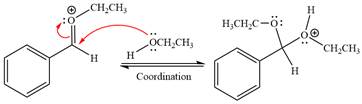
In the last step, the deprotonation of charged acetal by alcohol results in uncharged acetal
formation. Acetal is the major product of the given aldehyde.
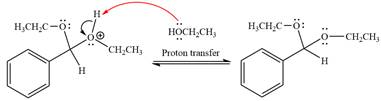
The complete, detailed mechanism of a given reaction in the acidic medium is shown below and an acetal is the major product.
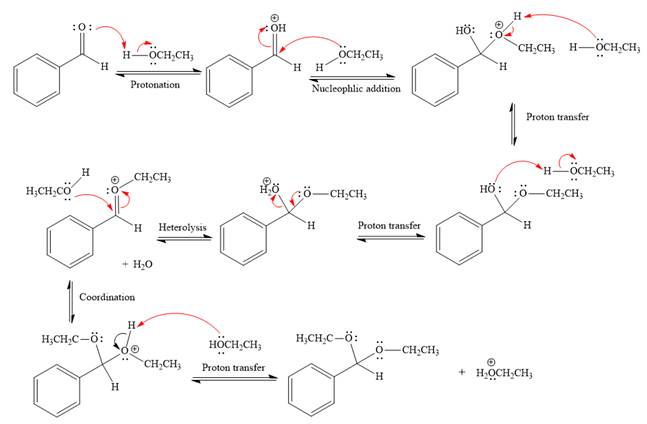
The complete, detailed mechanism of the given reaction under acidic medium and excess alcohol is drawn.
(b)
The complete, detailed mechanism of a given reaction in the acidic medium is to be drawn and the major organic product is to be predicted.
Concept introduction:
When an aldehyde or ketone is treated with an alcohol under acidic conditions, the hemiacetal product is formed. By using an excess amount of alcohol under acidic conditions, the nucleophilic addition produces hemiacetal, which further forms an acetal. The acetal has two alkoxy groups that are bonded to the same carbon. The formation of the acetal product is favored by using excess alcohol. This type of reaction is carried forward by proton transfer and nucleophilic addition on the carbonyl carbon. An acetal is produced under acidic conditions by a ketone or aldehyde but not under basic conditions because the nucleophilic substitution requires the leaving group
Answer to Problem 18.55P
The complete, detailed mechanism of a given reaction in the acidic medium is shown below and an acetal is a major product.
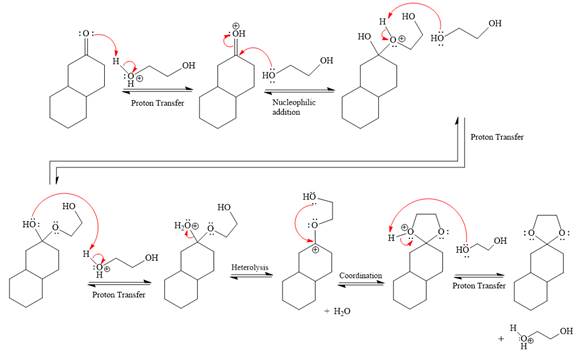
Explanation of Solution
The given reaction is
This is an acetal formation reaction in which the reaction is catalyzed by sulfuric acid and the excess alcohol (
First three steps are to the acid catalyze nucleophilic addition reactions on the ketone or aldehyde. In the first step, the
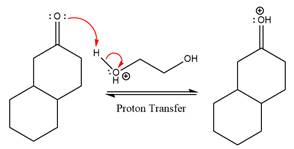
Next, the weak nucleophile,
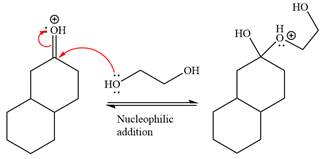
In the next step, deprotonation produces the uncharged hemiacetal.
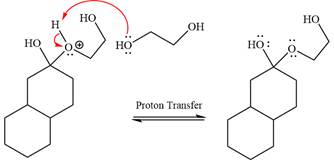
The remaining steps essentially make up a
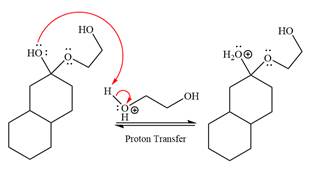
The
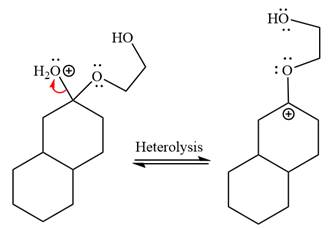
The resonance stabilized carbocation further attacked by the
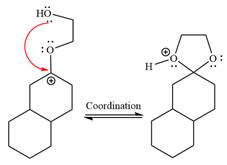
In the last step, the deprotonation of charged acetal by alcohol results in the uncharged acetal formation. Acetal is the major product of the given aldehyde.
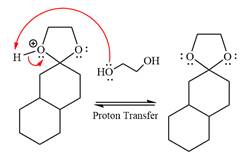
The complete, detailed mechanism of a given reaction in the acidic medium is shown below and an acetal is a major product.
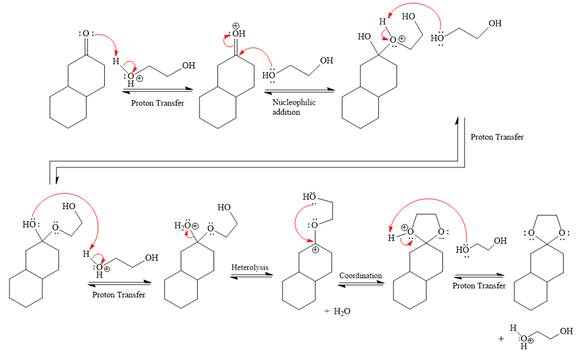
The complete, detailed mechanism of given reaction under acidic medium and excess alcohol is drawn.
(c)
Interpretation:
The complete, detailed mechanism of a given reaction in the acidic medium is to be drawn and major organic product is to be predicted.
Concept introduction:
When an aldehyde or ketone is treated with an alcohol under acidic conditions, the hemiacetal product is formed. By using an excess amount of alcohol under acidic conditions, the nucleophilic addition produces hemiacetal, which further forms an acetal. The acetal has two alkoxy groups are bonded to the same carbon. The formation of the acetal product is favored by using excess alcohol. This type of reactions carried forward by the proton transfer and nucleophilic addition on the carbonyl carbon. An acetal produced under acidic conditions by a ketone or aldehyde but not under basic conditions because the nucleophilic substitution that requires the leaving group to be
Answer to Problem 18.55P
The complete, detailed mechanism of a given reaction in the acidic medium is shown below and an acetal is a major product.
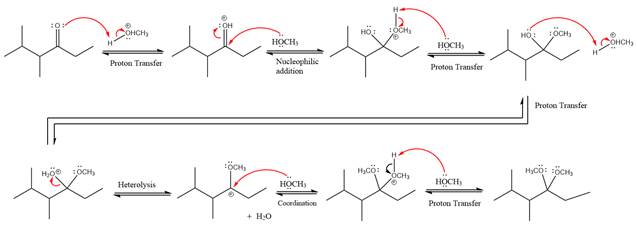
Explanation of Solution
The given reaction is
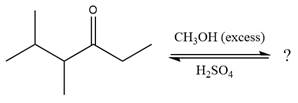
This is an acetal formation reaction in which the reaction is catalyzed by sulfuric acid and the excess methanol acts as the nucleophile.
First three steps are to the acid catalyze nucleophilic addition reactions on the ketone or aldehyde. In the first step, the
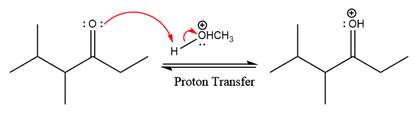
Next, the weak nucleophile, alcohol attacks on the activated electrophilic carbon by nucleophilic addition reaction.
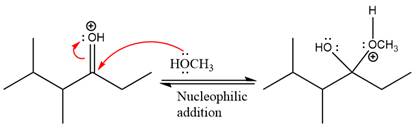
In the next step, deprotonation produces the uncharged hemiacetal.
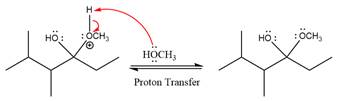
The remaining steps essentially make up a
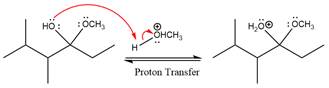
The
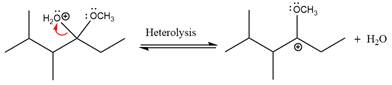
The resonance stabilized carbocation further attacked by the methyl alcohol nucleophile, which produced positively charged acetal.
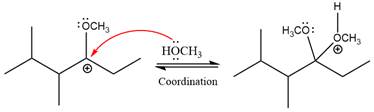
In the last step, the deprotonation of charged acetal by alcohol results in the uncharged acetal formation. Acetal is the major product of the given aldehyde.
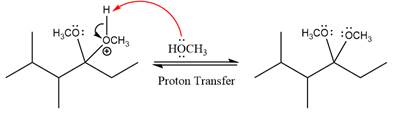
The complete, detailed mechanism of a given reaction in the acidic medium is shown below and an acetal is a major product.
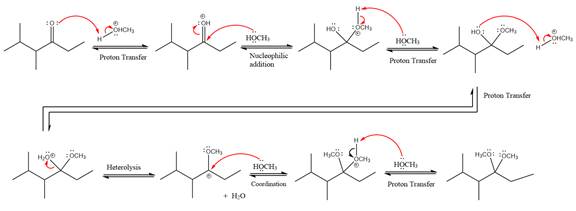
The complete, detailed mechanism of given reaction under acidic medium and excess alcohol is drawn.
(d)
Interpretation:
The complete, detailed mechanism of a given reaction in the acidic medium is to be drawn and major organic product is to be predicted.
Concept introduction:
When an aldehyde or ketone is treated with an alcohol under acidic conditions, the hemiacetal product is formed. By using an excess amount of alcohol under acidic conditions, the nucleophilic addition produces hemiacetal, which further forms an acetal. The acetal has two alkoxy groups are bonded to the same carbon. The formation of the acetal product is favored by using excess alcohol. This type of reactions carried forward by the proton transfer and nucleophilic addition on the carbonyl carbon. An acetal produced under acidic conditions by a ketone or aldehyde but not under basic conditions because the nucleophilic substitution that requires the leaving group to be
Answer to Problem 18.55P
The complete, detailed mechanism of a given reaction in the acidic medium is shown below and an acetal is a major product.
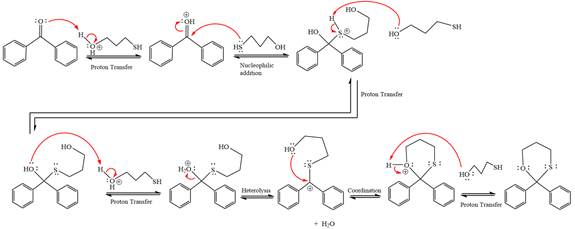
Explanation of Solution
The given reaction is
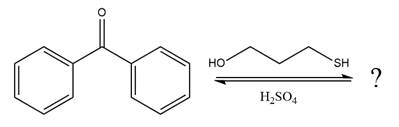
This is an acetal formation reaction in which the reaction is catalyzed by sulfuric acid and the excess
First three steps are to the acid catalyze nucleophilic addition reactions on the ketone or aldehyde. In the first step, the
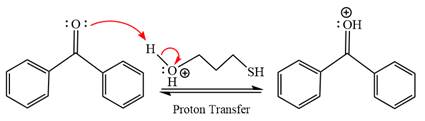
Next, the weak nucleophile, thiols attacks on the activated electrophilic carbon by nucleophilic addition reaction.
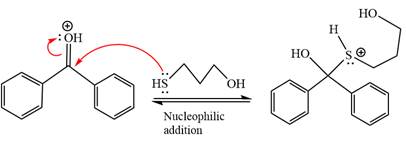
In the next step, deprotonation produces the uncharged hemiacetal.
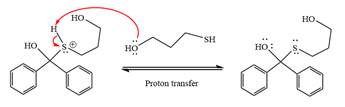
The remaining steps essentially make up a
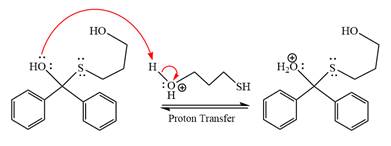
The
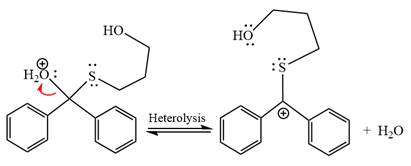
The resonance stabilized carbocation further attacked by the alcohol nucleophile, which produced positively charged acetal.
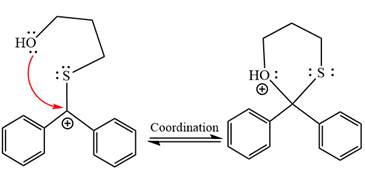
In the last step, the deprotonation of charged acetal by alcohol results in the uncharged acetal formation. Acetal is the major product of the given aldehyde.
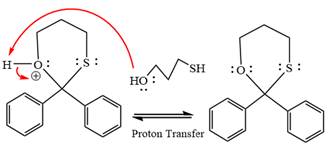
The complete, detailed mechanism of a given reaction in the acidic medium is shown below and an acetal is a major product.
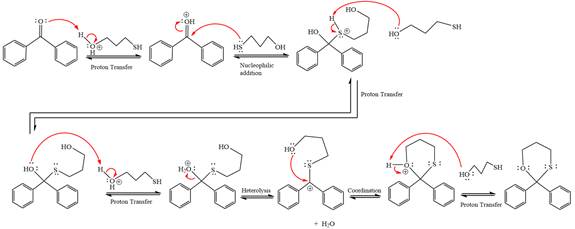
The complete, detailed mechanism of given reaction under acidic medium and excess alcohol is drawn.
(e)
Interpretation:
The complete, detailed mechanism of a given reaction in the acidic medium is to be drawn and major organic product is to be predicted.
Concept introduction:
When an aldehyde or ketone is treated with an alcohol under acidic conditions, the hemiacetal product is formed. By using an excess amount of alcohol under acidic conditions, the nucleophilic addition produces hemiacetal, which further forms an acetal. The acetal has two alkoxy groups are bonded to the same carbon. The formation of the acetal product is favored by using excess alcohol. This type of reactions carried forward by the proton transfer and nucleophilic addition on the carbonyl carbon. An acetal produced under acidic conditions by a ketone or aldehyde but not under basic conditions because the nucleophilic substitution that requires the leaving group to be
Answer to Problem 18.55P
The complete, detailed mechanism of a given reaction in the acidic medium is shown below and an acetal is a major product.
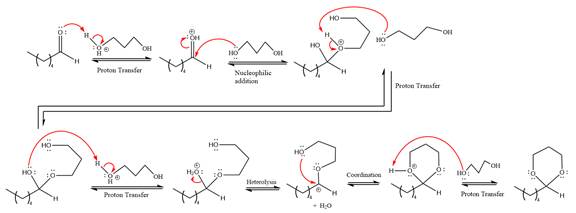
Explanation of Solution
The given reaction is
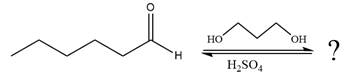
This is an acetal formation reaction in which the reaction is catalyzed by sulfuric acid and the excess
First three steps are to the acid catalyze nucleophilic addition reactions on the ketone or aldehyde. In the first step, the
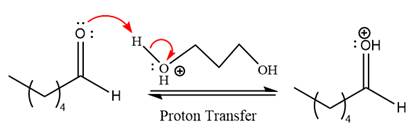
Next, the weak nucleophile,
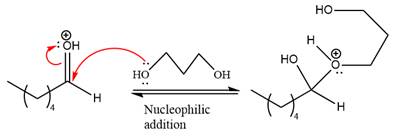
In the next step, deprotonation produces the uncharged hemiacetal.
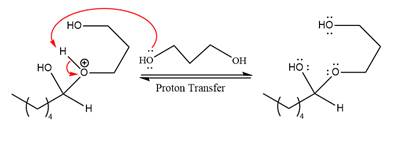
The remaining steps essentially make up a
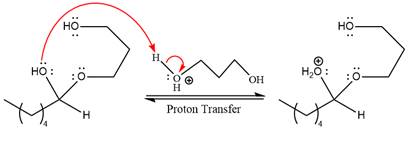
The
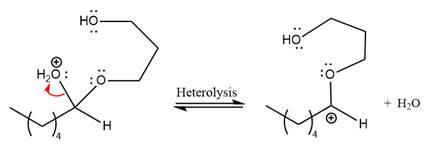
The resonance stabilized carbocation further attacked by the alcohol nucleophile, which produced positively charged acetal.
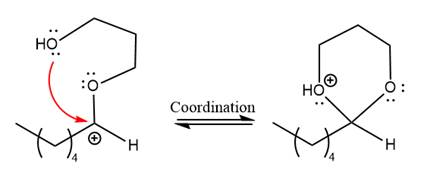
In the last step, the deprotonation of charged acetal by alcohol results in the uncharged acetal formation. Acetal is the major product of the given aldehyde.
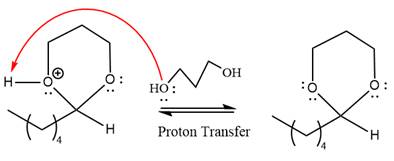
The complete, detailed mechanism of a given reaction in the acidic medium is shown below and an acetal is a major product.

The complete, detailed mechanism of given reaction under acidic medium and excess alcohol is drawn.
Want to see more full solutions like this?
Chapter 18 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- Draw the Markovnikov product of the hydrohalogenation of this alkene. this problem. Note for advanced students: draw only one product, and don't worry about showing any stereochemistry. Drawing dash and wedge bonds has been disabled for caps lock Explanation Check 2 W E R + X 5 HCI Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility Bil Y F G H K L ZZ X C V B N M control opption command F10 F10 command 4 BA Ar Carrow_forwardI don't understand why the amide on the top left, with the R attached to one side, doesn't get substituted with OH to form a carboxylic acid. And if only one can be substituted, why did it choose the amide it chose rather than the other amide?arrow_forwardesc Draw the Markovnikov product of the hydration of this alkene. Note for advanced students: draw only one product, and don't worry about showing any stereochemistry. Drawing dash and wedge bonds has been disabled for this problem. Explanation Check BBB + X 0 1. Hg (OAc)2, H₂O 2. Na BH 5 Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility Bl P 豆 28 2 28 N 9 W E R T Y A S aps lock G H K L Z X C V B N M T central H command #e commandarrow_forward
- C A student proposes the transformation below in one step of an organic synthesis. There may be one or more products missing from the right-hand side, but there are no reagents missing from the left-hand side. There may also be catalysts, small inorganic reagents, and other important reaction conditions missing from the arrow. • Is the student's transformation possible? If not, check the box under the drawing area. . If the student's transformation is possible, then complete the reaction by adding any missing products to the right-hand side, and adding required catalysts, inorganic reagents, or other important reaction conditions above and below the arrow. • You do not need to balance the reaction, but be sure every important organic reactant or product is shown. (X) This transformation can't be done in one step. + Tarrow_forwardく Predict the major products of this organic reaction. If there aren't any products, because nothing will happen, check the box under the drawing area instead. No reaction. Explanation Check OH + + ✓ 2 H₂SO 4 O xs H₂O 2 Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forwardDraw the skeletal ("line") structure of 1,3-dihydroxy-2-pentanone. Click and drag to start drawing a structure. X Parrow_forward
- Predicting edict the major products of this organic reaction. If there aren't any products, because nothing will happen, check the box under the drawing area instead. + No reaction. Explanation Check HO Na O H xs H₂O 2 Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Iarrow_forwardChoosing reagents and conditions for acetal formation or hydrolysis 0/5 A student proposes the transformation below in one step of an organic synthesis. There may be one or more products missing from the right-hand side, but there are no reagents missing from the left-hand side. There may also be catalysts, small inorganic reagents, and other important reaction conditions missing from the arrow. • Is the student's transformation possible? If not, check the box under the drawing area. If the student's transformation is possible, then complete the reaction by adding any missing products to the right-hand side, and adding required catalysts, inorganic reagents, or other important reaction conditions above and below the arrow. • You do not need to balance the reaction, but be sure every important organic reactant or product is shown. + This transformation can't be done in one step. 5 I H Autumn alo 值 Ar Barrow_forwardA block of copper of mass 2.00kg(cp = 0.3851 .K) and g temperature 0°C is introduced into an insulated container in which there is 1.00molH, O(g) at 100°C and 1.00 2 atm. Note that C P = 4.184. K for liquid water, and g that A H = 2260 for water. vap g Assuming all the steam is condensed to water, and that the pressure remains constant: (a) What will be the final temperature of the system? (b) What is the heat transferred from the water to the copper? (c) What is the entropy change of the water, the copper, and the total system?arrow_forward
- Identify the missing organic reactants in the following reaction: H+ X + Y OH H+ O O Note: This chemical equation only focuses on the important organic molecules in the reaction. Additional inorganic or small-molecule reactants or products (like H₂O) are not shown. In the drawing area below, draw the skeletal ("line") structures of the missing organic reactants X and Y. You may draw the structures in any arrangement that you like, so long as they aren't touching. Explanation Check Click and drag to start drawing a structure. X G 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Cente ? Earrow_forwardCalculate the solubility of CaF2 in g/L (Kp = 4.0 x 10-8). sparrow_forwardFor the following reaction with excess reagent, predict the product. Be sure your answer accounts for stereochemistry. If multiple stereocenters are formed, be sure to draw all products using appropriate wedges and dashes. 1. EtLi, Et₂O CH₁ ? 2. H₂O*arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





