
(a)
Interpretation:
The complete, detailed mechanism of a given reaction in mildly acidic medium is to be drawn and major organic product is to be predicted.
Concept introduction:
When a small amount of a strong acid is added to
Answer to Problem 18.57P
The complete, detailed mechanism of a given reaction in the acidic medium is shown below and an acetal is a major product.
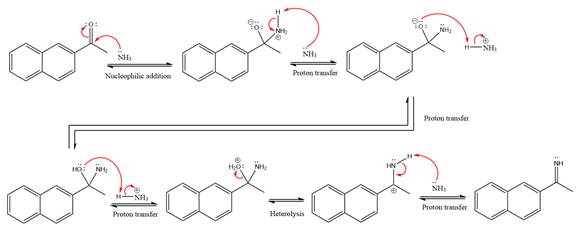
Explanation of Solution
The given reaction is
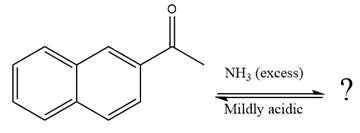
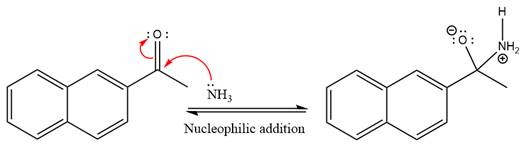
This is an imine formation reaction in which the reaction is catalyzed under mildly acidic condition and the excess ammonia acts as the nucleophile. Ammonia attacks the
First three steps are the nucleophilic addition reactions on the ketone. In the first step, nucleophile
In the next step, deprotonation takes place by ammonia
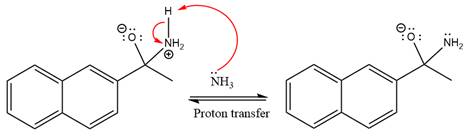
Next, negativively charged oxygen ion abstracts the proton from the ammonium ion.
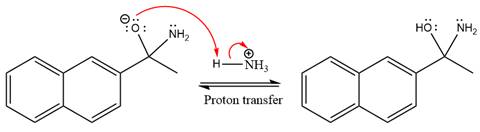
The lone pairs on
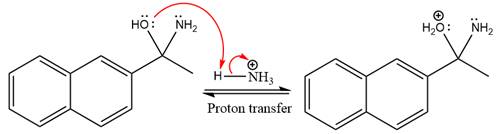
The
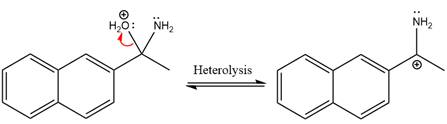
Ammonia nucleophile abstracts the proton from the amino group and forms imine as the product.
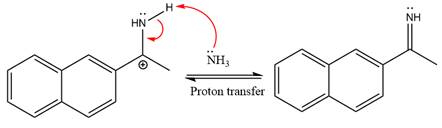
The complete, detailed mechanism of the given reaction under mildly acidic medium is shown below and an imine is the major product.
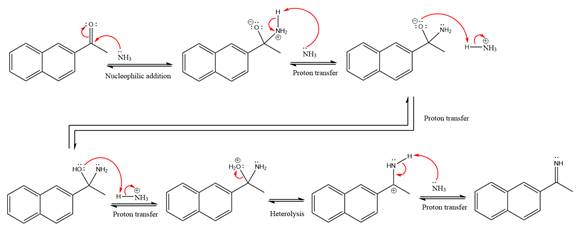
The complete, detailed mechanism of the given reaction under mildly acidic medium and excess ammonia is drawn.
(b)
Interpretation:
The complete, detailed mechanism of a given reaction in the acidic medium is to be drawn and major organic product is to be predicted.
Concept introduction:
When a small amount of a strong acid is added to ketone or an aldehyde, the uncharged nucleophile ammonia (
Answer to Problem 18.57P
The complete, detailed mechanism of the given reaction in the acidic medium is shown below and an acetal is the major product.
Explanation of Solution
The given reaction is
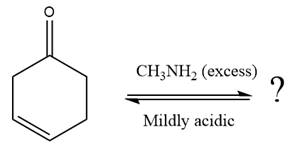
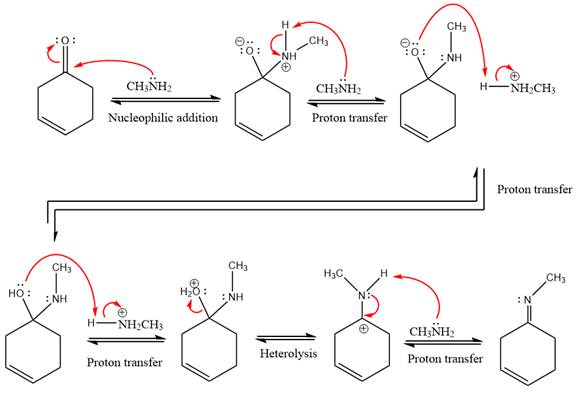
This is an imine formation reaction in which the reaction is catalyzed under mildly acidic condition and excess methylamine acts as the nucleophile.
First three steps are nucleophilic addition reactions on ketone. In the first step, nucleophile
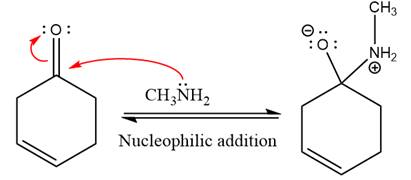
In the next step, deprotonation takes place by the ammonia
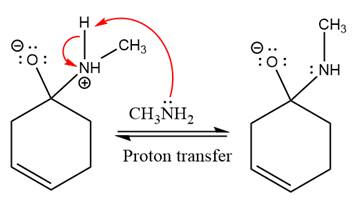
Next, negativively charged oxygen ion abstracts the proton from methylamino ion.
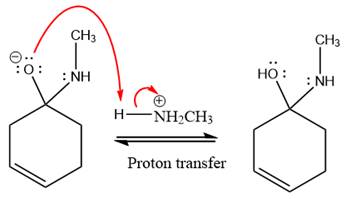
The lone pairs on
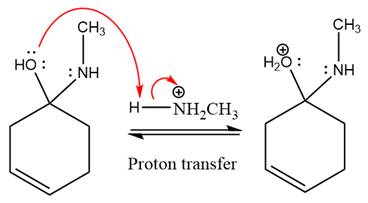
The
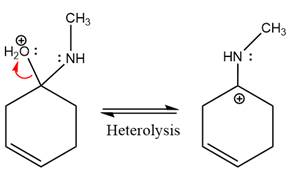
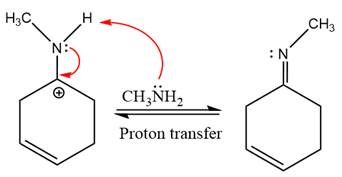
The complete, detailed mechanism of the given reaction under mildly acidic medium is shown below and an imine is the major product.
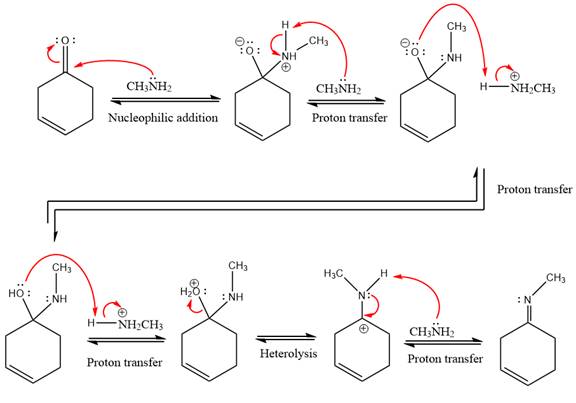
The complete, detailed mechanism of the given reaction under mildly acidic medium and excess methylamine is drawn.
(c)
Interpretation:
The complete, detailed mechanism of a given reaction under acidic medium is to be drawn and major organic product is to be predicted.
Concept introduction:
When a small amount of a strong acid is added to ketone or an aldehyde, the uncharged nucleophile ammonia (
Answer to Problem 18.57P
The complete, detailed mechanism of the given reaction in the acidic medium is shown below and an ketone is the major product.
Explanation of Solution
The given reaction is
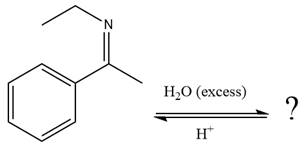
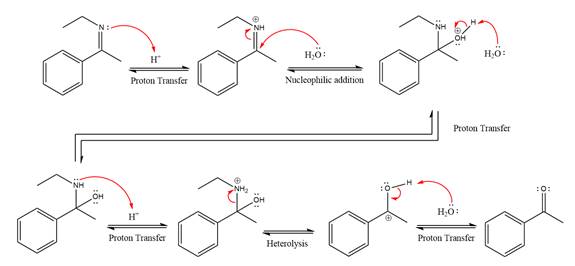
This is an imine hydrolysis reaction under strongly acidic condition to produce ketone as the major product.
In the first step, the
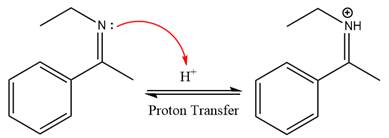
Next, water nucleophile attacks the activated electrophilic carbon by nucleophilic addition reaction.
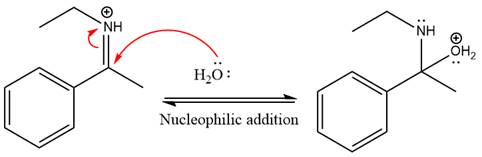
In the next step, deprotonation takes place by the nucleophile produced
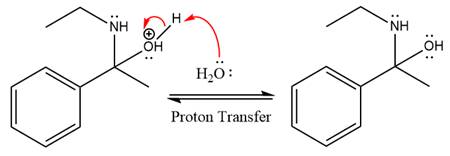
The lone pairs on
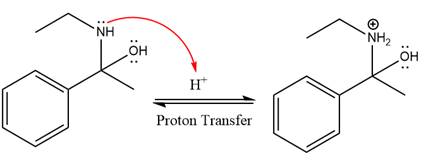
The
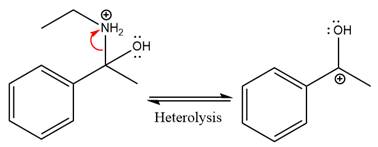
The nucleophile water abstracts the proton in the resonance stabilized carbocation and produces ketone as the major product.
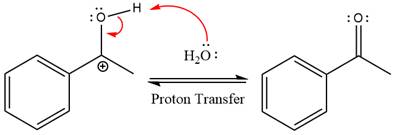
The complete, detailed mechanism of the given reaction under strongly acidic medium is shown below and ketone is the major product.
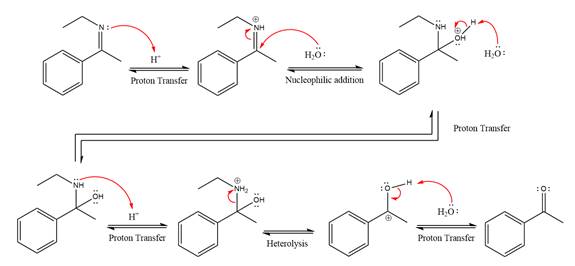
The complete, detailed mechanism of the given reaction under strongly acidic medium and excess water is drawn.
(d)
Interpretation:
The complete, detailed mechanism of the given reaction in the mildly acidic medium is to be drawn and major organic product is to be predicted.
Concept introduction:
When a small amount of a strong acid is added to ketone or an aldehyde, the uncharged nucleophile ammonia (
Answer to Problem 18.57P
The complete, detailed mechanism of the given reaction in mildly acidic medium is shown below and enamine is the major product.
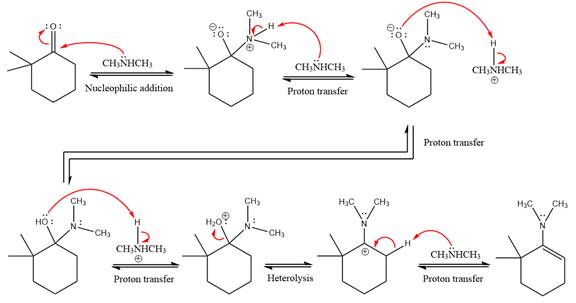
Explanation of Solution
The given reaction is
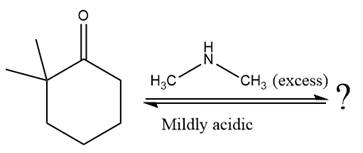
This is an enamine formation reaction in which the reaction is catalyzed under mildly acidic conditions, and the secondary
First three steps are nucleophilic addition reactions on ketone. In the first step, nucleophile
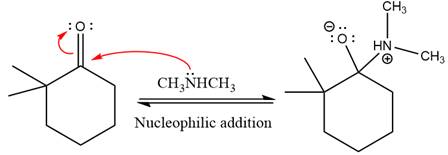
In the next step, deprotonation takes place by
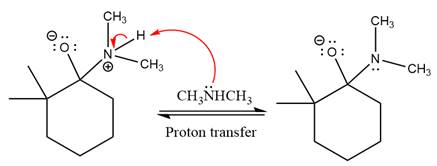
Next, negatively charged oxygen ion abstracts the proton from dimethylamino ion.
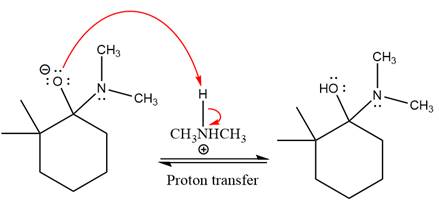
The lone pairs on
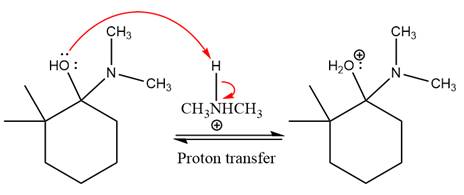
The
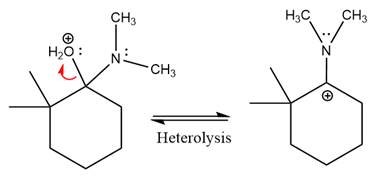
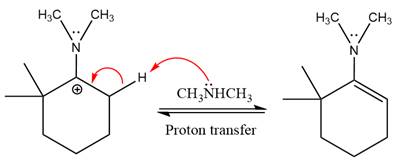
The complete, detailed mechanism of the given reaction in mildly acidic medium is shown below and enamine is the major product.

The complete, detailed mechanism of the given reaction under acidic medium and excess alcohol is drawn.
(e)
Interpretation:
The complete, detailed mechanism of a given reaction under strongly acidic medium is to be drawn and major organic product is to be predicted.
Concept introduction:
When a small amount of a strong acid is added to ketone or an aldehyde, the uncharged nucleophile
Answer to Problem 18.57P
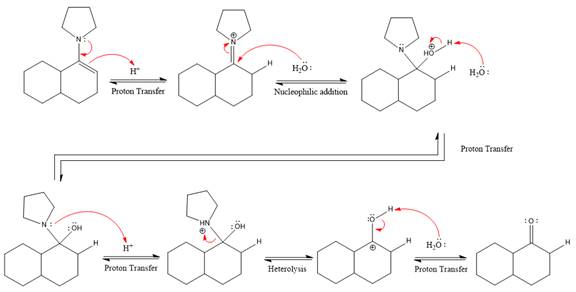
The complete, detailed mechanism of the given reaction under strongly acidic medium is shown below and ketone is the major product.
Explanation of Solution
The given reaction is
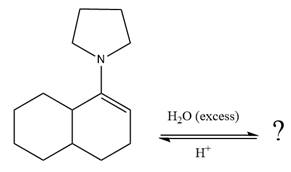
This is an enamine hydrolysis reaction under strongly acidic condition to produce ketone as the major product.
In the first step,
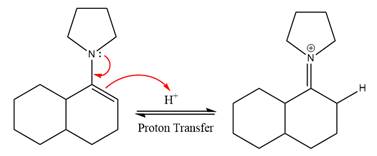
Next, water nucleophile attacks the activated electrophilic carbon by nucleophilic addition reaction.
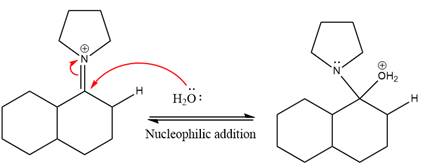
In the next step, deprotonation takes place by the nucleophile produced
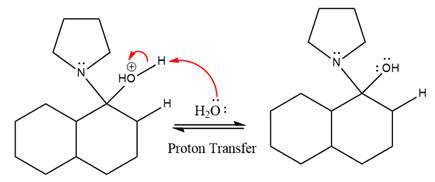
The lone pairs on
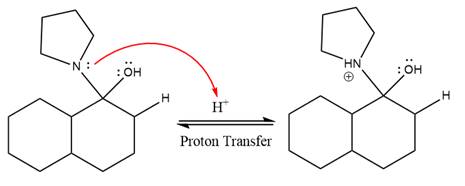
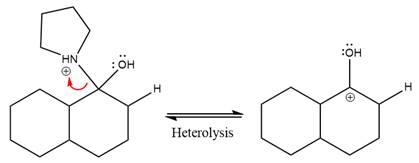
The nucleophile water abstracts the proton in the resonance stabilized carbocation and produces ketone as the major product.
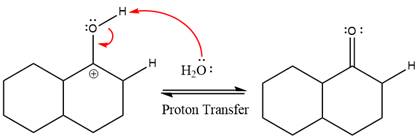
The complete, detailed mechanism of the given reaction in the acidic medium is shown below and an acetal is the major product.
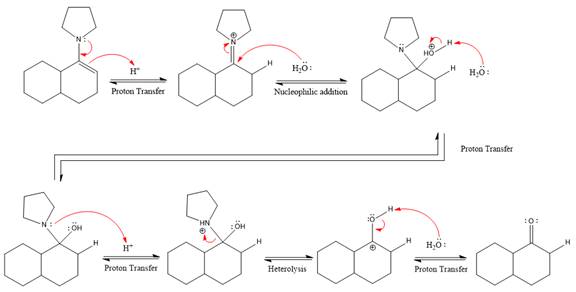
The complete, detailed mechanism of the given reaction under strongly acidic medium and excess water is drawn.
Want to see more full solutions like this?
Chapter 18 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- HI Organic Functional Groups Predicting the reactants or products of esterification What is the missing reactant in this organic reaction? HO OH H +回 + H₂O 60013 Naomi V Specifically, in the drawing area below draw the skeletal ("line") structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. No answer Click and drag to start drawing a structure. Explanation Check 1 2 #3 $ 4 2025 % ala5 'a :☐ G & 67 8 Ar K enter Accessible 9 Q W E R TY U 1 tab , S H J Karrow_forwardPlease help me with number 5 using my data and graph. I think I might have number 3 and 4 but if possible please check me. Thanks in advance!arrow_forwarddict the major products of this organic reaction. C Explanation Check 90 + 1.0₂ 3 2. (CH3)2S Click and drag f drawing a stru © 2025 McGraw Hill LLC. All Rights Reserved. • 22 4 5 7 8 Y W E R S F H Bilarrow_forward
- can someone draw out the reaction mechanism for this reaction showing all the curly arrows and 2. Draw the GPNA molecule and identify the phenylalanine portion. 3. Draw L-phenylalanine with the correct stereochemistryarrow_forwardWhat is the reaction mechanism for this?arrow_forwardPredict the major products of both organic reactions. Be sure to use wedge and dash bonds to show the stereochemistry of the products when it's important, for example to distinguish between two different major products. esc esc Explanation Check 2 : + + X H₁₂O + Х ง WW E R Y qab Ccaps lock shift $ P X Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility Bil T FR F18 9 G t K L Z X V B N M control opption command command T C darrow_forward
- Draw the Markovnikov product of the hydrohalogenation of this alkene. this problem. Note for advanced students: draw only one product, and don't worry about showing any stereochemistry. Drawing dash and wedge bonds has been disabled for caps lock Explanation Check 2 W E R + X 5 HCI Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility Bil Y F G H K L ZZ X C V B N M control opption command F10 F10 command 4 BA Ar Carrow_forwardI don't understand why the amide on the top left, with the R attached to one side, doesn't get substituted with OH to form a carboxylic acid. And if only one can be substituted, why did it choose the amide it chose rather than the other amide?arrow_forwardesc Draw the Markovnikov product of the hydration of this alkene. Note for advanced students: draw only one product, and don't worry about showing any stereochemistry. Drawing dash and wedge bonds has been disabled for this problem. Explanation Check BBB + X 0 1. Hg (OAc)2, H₂O 2. Na BH 5 Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibility Bl P 豆 28 2 28 N 9 W E R T Y A S aps lock G H K L Z X C V B N M T central H command #e commandarrow_forward
- C A student proposes the transformation below in one step of an organic synthesis. There may be one or more products missing from the right-hand side, but there are no reagents missing from the left-hand side. There may also be catalysts, small inorganic reagents, and other important reaction conditions missing from the arrow. • Is the student's transformation possible? If not, check the box under the drawing area. . If the student's transformation is possible, then complete the reaction by adding any missing products to the right-hand side, and adding required catalysts, inorganic reagents, or other important reaction conditions above and below the arrow. • You do not need to balance the reaction, but be sure every important organic reactant or product is shown. (X) This transformation can't be done in one step. + Tarrow_forwardく Predict the major products of this organic reaction. If there aren't any products, because nothing will happen, check the box under the drawing area instead. No reaction. Explanation Check OH + + ✓ 2 H₂SO 4 O xs H₂O 2 Click and drag to start drawing a structure. © 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Centerarrow_forwardDraw the skeletal ("line") structure of 1,3-dihydroxy-2-pentanone. Click and drag to start drawing a structure. X Parrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





