
Introduction To General, Organic, And Biochemistry
12th Edition
ISBN: 9781337571357
Author: Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 6P
17-14 Following are structural formulas for two steroid hormones.
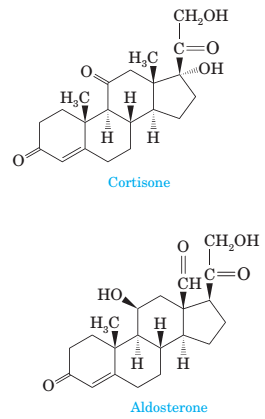
(a) Name the functional groups in each.
(b) Mark all stereocenters in each hormone and state how many stereoisomers are possible for each.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Which of the following are descriptions of possible starting material for this
reaction?
H
?
trace acid
an ester
a ketone
an imine
an aldehyde
a carboxylic acid
an enamine
a primary amine
a secondary amine
a tertiary amine
None
What are the reagents needed for this and the third structure I only got the top right structure right
Chapter 16 Solutions
Introduction To General, Organic, And Biochemistry
Ch. 16.2 - Problem 17-1 Wrtie the IUPAC name for each...Ch. 16.2 - Prob. 16.2QCCh. 16.2 - Prob. 16.3QCCh. 16.4 - Prob. 16.4QCCh. 16.4 - Prob. 16.5QCCh. 16.4 - Problem 17-6 Show the reaction of benzaldehyde...Ch. 16.4 - Problem 17-7 Identify all hemiacetals and acetals...Ch. 16.5 - Prob. 16.8QCCh. 16 - 17-9 Answer true or false. (a) The one aldehyde...Ch. 16 - Prob. 2P
Ch. 16 - 17-11 What is the difference in structure between...Ch. 16 - 17-12 Is it possible for the carbon atom of a...Ch. 16 - 17-13 Which compounds contain carbonyl groups?Ch. 16 - 17-14 Following are structural formulas for two...Ch. 16 - 17-15 Draw structural formulas for the four...Ch. 16 - Prob. 8PCh. 16 - Prob. 9PCh. 16 - 17-18 Draw structural formulas for these ketones....Ch. 16 - 17-19 Write the JUPAC names for these compounds.Ch. 16 - Prob. 12PCh. 16 - 17-2 1 Explain why each name is incorrect. Write...Ch. 16 - Prob. 14PCh. 16 - Prob. 15PCh. 16 - 17-24 In each pair of compounds, select the one...Ch. 16 - Prob. 17PCh. 16 - 17-26 Account for the fact that acetone has a...Ch. 16 - 17-27 Pentane, 1-butanol, and butanal all have...Ch. 16 - 17-28 Show how acetaldehyde can form hydrogen...Ch. 16 - 17-29 Why can’t two molecules of acetone form a...Ch. 16 - 17-30 Answer true or false. (a) The reduction of...Ch. 16 - 17-3 1 Draw a structural formula for the principal...Ch. 16 - Prob. 24PCh. 16 - 17-33 What simple chemical test could you use to...Ch. 16 - 17-34 Explain why liquid aldehydes are often...Ch. 16 - 17-35 Suppose that you take a bottle of...Ch. 16 - 17-36 Explain why the reduction of an aldehyde...Ch. 16 - Prob. 29PCh. 16 - Prob. 30PCh. 16 - Prob. 31PCh. 16 - Prob. 32PCh. 16 - Prob. 33PCh. 16 - Prob. 34PCh. 16 - Prob. 35PCh. 16 - Prob. 36PCh. 16 - Prob. 37PCh. 16 - Prob. 38PCh. 16 - 17-47 What is the characteristic structural...Ch. 16 - Prob. 40PCh. 16 - Prob. 41PCh. 16 - Prob. 42PCh. 16 - Prob. 43PCh. 16 - Prob. 44PCh. 16 - Prob. 45PCh. 16 - 17-54 Following is the structure of...Ch. 16 - Prob. 47PCh. 16 - Prob. 48PCh. 16 - Prob. 49PCh. 16 - Prob. 50PCh. 16 - Prob. 51PCh. 16 - 17-60 1-Propanol can be prepared by the reduction...Ch. 16 - Prob. 53PCh. 16 - 17-62 Show how to bring about these conversions....Ch. 16 - Prob. 55PCh. 16 - Prob. 56PCh. 16 - Prob. 57PCh. 16 - Prob. 58PCh. 16 - 17-67 Draw structural formulas for these...Ch. 16 - Prob. 60PCh. 16 - 17-69 Propanal (bp 49°C) and 1-propanol (bp 97°C)...Ch. 16 - 17-70 What simple chemical test could you use to...Ch. 16 - Prob. 63PCh. 16 - Prob. 64PCh. 16 - Prob. 65PCh. 16 - 17-72 The following molecule is an enediol; each...Ch. 16 - 17-73 Alcohols can be prepared by the...Ch. 16 - 17-74 Glucose, C6H12O6, contains an aldehyde group...Ch. 16 - Prob. 69PCh. 16 - Prob. 70PCh. 16 - Prob. 71PCh. 16 - 17-78 Complete the following equation for these...Ch. 16 - 17-79 Write an equation for each conversion. (a)...Ch. 16 - Prob. 74P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please label this COZY spectraarrow_forwardPlease label this HNMRarrow_forwardConsider the following gas chromatographs of Compound A, Compound B, and a mixture of Compounds A and B. Inject A B mixture Area= 9 Area = 5 Area = 3 Area Inject . མི། Inject J2 What is the percentage of Compound B in the the mixture?arrow_forward
- Rank these according to stability. CH3 H3C CH3 1 CH3 H3C 1 most stable, 3 least stable O 1 most stable, 2 least stable 2 most stable, 1 least stable O2 most stable, 3 least stable O3 most stable, 2 least stable O3 most stable, 1 least stable CH3 2 CH3 CH3 H₂C CH3 3 CH3 CHarrow_forwardConsider this IR and NMR: INFRARED SPECTRUM TRANSMITTANCE 0.8- 0.6 0.4 0.2 3000 10 9 8 00 HSP-00-541 7 CO 6 2000 Wavenumber (cm-1) сл 5 ppm 4 M Which compound gave rise to these spectra? N 1000 1 0arrow_forwardConsider this reaction (molecular weights are under each compound): HC=CH + 2 HCI --> C2H4Cl 2 MW = 26 36.5 99 If 4.4 g of HC=CH are reacted with 110 mL of a 2.3 M HCI solution, and 6.0 g of product are actually produced, what is the percent yield?arrow_forward
- What is the name of the major product of this reaction? OH CH3 H₂SO4, heat 1-methylcyclohexene O2-methyl-1-cyclohexene O 3-mthylcyclohexene 1-methyl-2-cyclohexenearrow_forwardWe added a brown solution of Br2 to one of our products, and the brown color disappeared. This indicated that our product wasarrow_forwardRank the following according to reactivity toward nitration: a) benzene b) bromobenzene c) nitrobenzene d) phenol Od) greatest, c) least Od) greatest, b) least Od) greatest, a) least a) greatest, b) least a) greatest, c) least Oa) greatest, d) least Ob) greatest, a) least O b) greatest, c) least Ob) greatest, d) least O c) greatest, a) least O c) greatest, b) least O c) greatest, d) leastarrow_forward
- O-Nitrophenol was distilled over with the steam in our experiment while the other isomer did not. This is due to: O intramolecular hydrogen bonding in the ortho isomer O intermolecular hydrogen bonding in the the ortho isomer O the ortho isomer has a lower density O the ortho isomer has a lower molecular weightarrow_forwardK 44% Problem 68 of 15 Submit Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. :6: :: :CI: CI CI: :0:0 Select to Add Arrows Select to Add Arrows H H Cl CI: CI CI: Select to Add Arrows Select to Add Arrows H :CI: Alarrow_forwardI I H :0: Submit Curved arrows are used to illustrate the flow of electrons. Using the provided starting and product structures, draw the curved electron-pushing arrows for the following reaction or mechanistic step(s). Be sure to account for all bond-breaking and bond-making steps. 0:0 :0: CI ΑΙ :CI: :CI: :0: CI Select to Add Arrows Select to Add Arrows cl. :0: Cl © ハ CI:: CI H CO Select to Add Arrows Select to Add Arrows 10: AI ::arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Brooks Cole
Lipids - Fatty Acids, Triglycerides, Phospholipids, Terpenes, Waxes, Eicosanoids; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=7dmoH5dAvpY;License: Standard YouTube License, CC-BY