
(a)
Interpretation:
To draw the structural formula for an
Concept Introduction:
Generally an aldehyde or ketone reduced to give the corresponding 1° or 2° alcohol. There are several types of reducing agents such as Pd /H2, NaBH4, LiAlH4 etc.
Mechanism for NaBH4 reduction explained below.
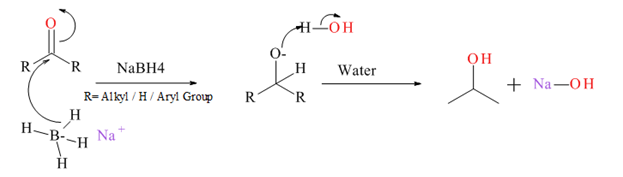
(b)
Interpretation:
To draw the structural formula for an aldehyde or ketone that can be reduced to form given alcohol.
Concept Introduction:
Generally an aldehyde or ketone reduced to give the corresponding 1° or 2° alcohol. There are several types of reducing agents such as Pd /H2, NaBH4, LiAlH4 etc.
Mechanism for NaBH4 reduction explained below.
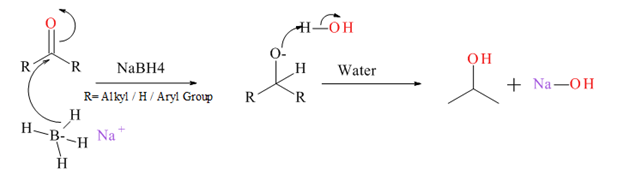
(c)
Interpretation:
To draw the structural formula for an aldehyde or ketone that can be reduced to form given alcohol.
Concept Introduction:
Generally an aldehyde or ketone reduced to give the corresponding 1° or 2° alcohol. There are several types of reducing agents such as Pd /H2, NaBH4, LiAlH4 etc.
Mechanism for NaBH4 reduction explained below.
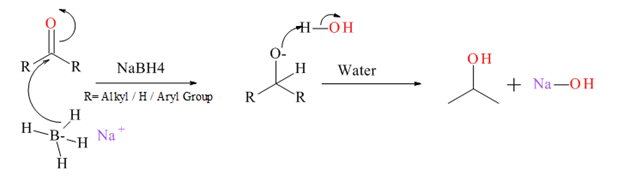
(d)
Interpretation:
To draw the structural formula for an aldehyde or ketone that can be reduced to form given alcohol.
Concept Introduction:
Generally an aldehyde or ketone reduced to give the corresponding 1° or 2° alcohol. There are several types of reducing agents such as Pd /H2, NaBH4, LiAlH4 etc.
Mechanism for NaBH4 reduction explained below.
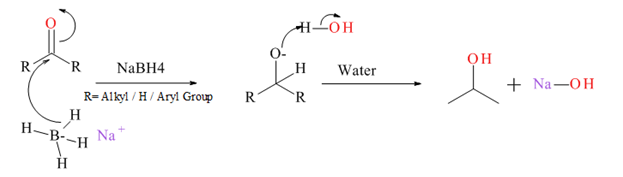
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
Introduction To General, Organic, And Biochemistry
- Which synthesis of a Grignard reagent would fail to occur as written? Why is the correct answer D? Please explain what is happening. Please include a detailed explanation and a drawing of steps needed to understand the reaction or question.arrow_forwardDraw the structure of the major organic product(s) of the reaction. H3C 1. DIBAH, toluene -CH3 + 2. H3O DIBAH = diisobutylaluminum hydride, [(CH3)2CHCH2]2AIHarrow_forwardWhich of the following is not an intermediate of the reaction below? Why is the correct answer C? Please explain what is happening. Please include a detailed explanation and a drawing of steps needed to understand the reaction or question.arrow_forward
- Which of the following is the product of the reaction between acetone, CH3COCH3 and methyl amine, CH3NH2? Why is the correct answer A? Please explain what is happening. Please include a detailed explanation and a drawing of steps needed to understand the reaction or question.arrow_forwardWhat is the product of the reaction shown below? Why is the correct answer D? Please explain what is happening. Please include a detailed explanation and a drawing of steps needed to understand the reaction or question.arrow_forwardWrite the systematic name of each organic molecule: structure name П O ☐ O ☐ Oarrow_forward
- The 13C NMR signal for which of the indicated carbons will occur at the frequency (most deshielded)? Why is the correct answer E? Please explain what is happening. Please include a detailed explanation needed to understand the or question.arrow_forwardWhich of the following reagents best achieves the reaction shown below? Why is the correct answer B? Please explain what is happening. Please include a detailed explanation and a drawing of steps needed to understand the reaction or question.arrow_forwardWhat is the product of the following reaction sequence? Why is the correct answer D? Please explain what is happening. Please include a detailed explanation and a drawing of steps needed to understand the reaction or question.arrow_forward
- Pls help ASAParrow_forwardThe reaction of phenylmagnesium bromide (C6H5MgBr) with propanal (CH3CH2CHO)3 followed by hydrolysis yields. A. 2-phenyl-1-propanol B. 1-phenyl-1propanol C. 3-phenyl-2-propanol D. 3-phenyl-1-propanol Why is the correct answer B? Please explain what is happening. Please include a detailed explanation and/or a drawing of steps needed to understand the reaction or question.arrow_forwardWhat is the product of the reaction sequence below? Why is the correct answer D? Please explain what is happening. Please include a detailed explanation and a drawing of steps needed to understand the reaction or question. The part that is under the pen in the image is (1) CH3CHO (2) H3O+arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning





