
Concept explainers
17-73 Alcohols can be prepared by the acid-catalyzed hydration of
(a) Ethanol
(b) Cyclohexanol
(c) 2-Propanol
(d) 1-Phenylethanol
(a)
Interpretation:
Show the preparation of ethanol by acid-catalyzed hydration of an alkene and by reduction of an aldehyde or a ketone.
Concept Introduction:
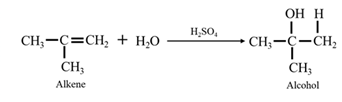
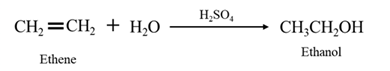
Acid-catalyzed hydration of alkenes: In the presence of an acid catalyst
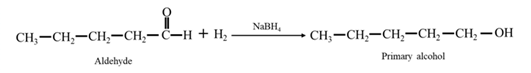
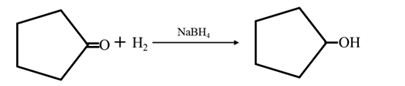
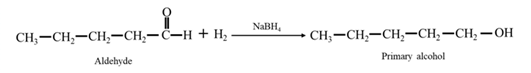
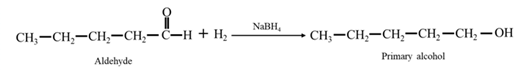
Reduction of an aldehyde or a ketone: The C=C double bond of an alkene is reduced by hydrogen in the presence of a transition metal catalyst to a C−C single bond. The same is true for the C=O double bond of an aldehyde or a ketone. Aldehydes are reduced to primary alcohols and ketones are reduced to secondary alcohol.
Answer to Problem 67P
By acid-catalyzed hydration of ethane:
By Reduction of ethanal:
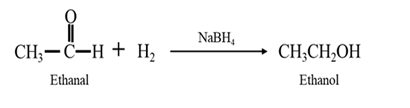
Explanation of Solution
By acid-catalyzed hydration of ethane:
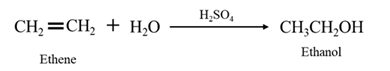
When ethene is allowed to react with water in presence of an acid catalyst it gives ethanol.
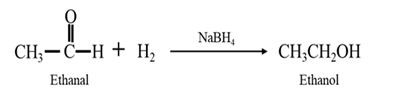
By Reduction of ethanal: When ethanal is reduced in the presence of sodium borohydride it gives ethanol.
(b)
Interpretation:
Show the preparation of cyclohexanol by acid-catalyzed hydration of an alkene and by reduction of an aldehyde or a ketone.
Concept Introduction:
Acid-catalyzed hydration of alkenes: In the presence of an acid catalyst
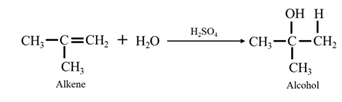
Reduction of an aldehyde or a ketone: The C=C double bond of an alkene is reduced by hydrogen in the presence of a transition metal catalyst to a C−C single bond. The same is true for the C=O double bond of an aldehyde or a ketone. Aldehydes are reduced to primary alcohols and ketones are reduced to secondary alcohol.
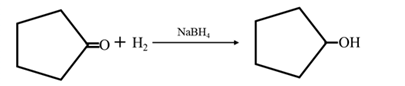
Answer to Problem 67P
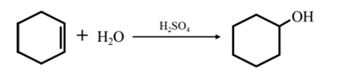
By acid-catalyzed hydration of ethane:
By Reduction of ethanal:
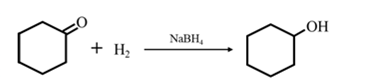
Explanation of Solution
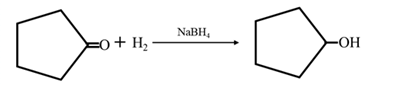
By acid-catalyzed hydration of ethane: When cyclohexene is allowed to react with water in presence of an acid catalyst it gives cyclohexanol.
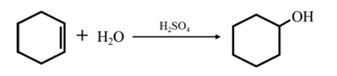
By Reduction of ethanal: When cyclohexanone is reduced in the presence of sodium borohydride it gives cyclohexanol.
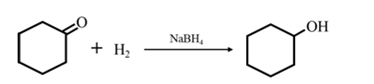
(c)
Interpretation:
Show the preparation of 2-propanol by acid-catalyzed hydration of an alkene and by reduction of an aldehyde or a ketone.
Concept Introduction:
Acid-catalyzed hydration of alkenes: In the presence of an acid catalyst
Reduction of an aldehyde or a ketone: The C=C double bond of an alkene is reduced by hydrogen in the presence of a transition metal catalyst to a C−C single bond. The same is true for the C=O double bond of an aldehyde or a ketone. Aldehydes are reduced to primary alcohols and ketones are reduced to secondary alcohol.
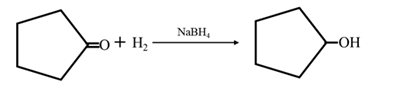
Answer to Problem 67P
By acid-catalyzed hydration of ethane:
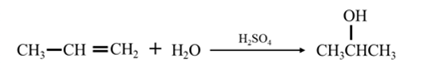
By Reduction of ethanal:
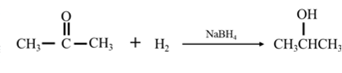
Explanation of Solution
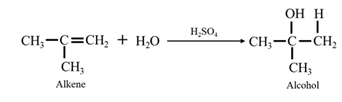
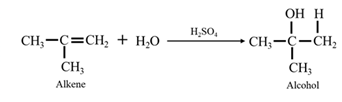
By acid-catalyzed hydration of ethane: When propene is allowed to react with water in presence of an acid catalyst it gives 2-propanol.
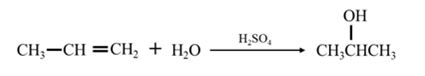
By Reduction of ethanal: When acetone is reduced in the presence of sodium borohydride it gives 2-propanol.
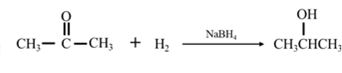
(d)
Interpretation:
Show the preparation of 1-phenylethanol by acid-catalyzed hydration of an alkene and by reduction of an aldehyde or a ketone.
Concept Introduction:
Acid-catalyzed hydration of alkenes: In the presence of an acid catalyst
Reduction of an aldehyde or a ketone: The C=C double bond of an alkene is reduced by hydrogen in the presence of a transition metal catalyst to a C−C single bond. The same is true for the C=O double bond of an aldehyde or a ketone. Aldehydes are reduced to primary alcohols and ketones are reduced to secondary alcohol.
Answer to Problem 67P
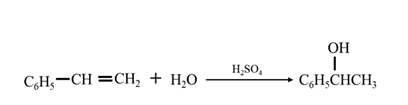
By acid-catalyzed hydration of ethane:
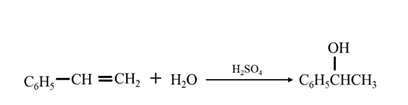
By Reduction of ethanal:
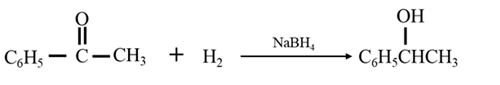
Explanation of Solution
By acid-catalyzed hydration of ethane: When 1-phenylethene is allowed to react with water in presence of an acid catalyst it gives 1-phenylethanol.
By Reduction of ethanal: When acetophenone is reduced in the presence of sodium borohydride it gives 1-phenylethanol.
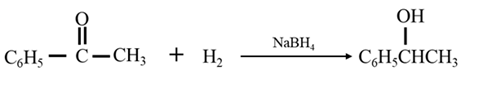
Want to see more full solutions like this?
Chapter 16 Solutions
Introduction To General, Organic, And Biochemistry
- curved arrows are used to illustrate the flow of electrons. using the provided starting and product structures, draw the cured electron-pushing arrows for thw following reaction or mechanistic steps. be sure to account for all bond-breaking and bond making stepsarrow_forwardUsing the graphs could you help me explain the answers. I assumed that both graphs are proportional to the inverse of time, I think. Could you please help me.arrow_forwardSynthesis of Dibenzalacetone [References] Draw structures for the carbonyl electrophile and enolate nucleophile that react to give the enone below. Question 1 1 pt Question 2 1 pt Question 3 1 pt H Question 4 1 pt Question 5 1 pt Question 6 1 pt Question 7 1pt Question 8 1 pt Progress: 7/8 items Que Feb 24 at You do not have to consider stereochemistry. . Draw the enolate ion in its carbanion form. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. ⚫ Separate multiple reactants using the + sign from the drop-down menu. ? 4arrow_forward
- Shown below is the mechanism presented for the formation of biasplatin in reference 1 from the Background and Experiment document. The amounts used of each reactant are shown. Either draw or describe a better alternative to this mechanism. (Note that the first step represents two steps combined and the proton loss is not even shown; fixing these is not the desired improvement.) (Hints: The first step is correct, the second step is not; and the amount of the anhydride is in large excess to serve a purpose.)arrow_forwardHi I need help on the question provided in the image.arrow_forwardDraw a reasonable mechanism for the following reaction:arrow_forward
- Draw the mechanism for the following reaction: CH3 CH3 Et-OH Et Edit the reaction by drawing all steps in the appropriate boxes and connecting them with reaction arrows. Add charges where needed. Electron-flow arrows should start on the electron(s) of an atom or a bond and should end on an atom, bond, or location where a new bond should be created. H± EXP. L CONT. י Α [1] осн CH3 а CH3 :Ö Et H 0 N о S 0 Br Et-ÖH | P LL Farrow_forward20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCl. What is the molarity of the HCl?arrow_forward20.00 mL of 0.025 M HCl is titrated with 0.035 M KOH. What volume of KOH is needed?arrow_forward
- 20.00 mL of 0.150 M NaOH is titrated with 37.75 mL of HCl. What is the molarity of the HCl?arrow_forward20.00 mL of 0.025 M HCl is titrated with 0.035 M KOH. What volume of KOH is needed?arrow_forward20.00 mL of 0.150 M HCl is titrated with 37.75 mL of NaOH. What is the molarity of the NaOH?arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


