
(a)
Interpretation:
To classify the given compounds as hemi-acetal, acetal and neithers.
Concept Introduction:
Hemiacetal is a type of molecule which contains carbon bonded to one -OH group and one -OR group. It is basically a half acetal. A hemiacetal forms when one molecule of alcohol reacts with a carbonyl group of an
For an Example:
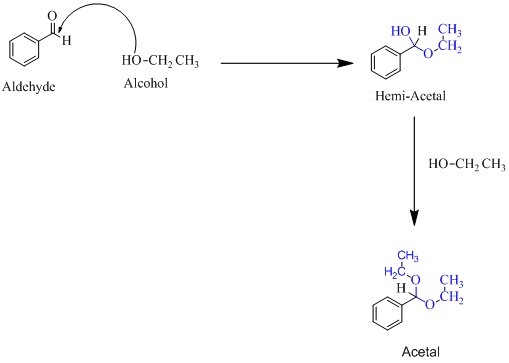
(b)
Interpretation:
To classify the given compounds as hemi-acetal, acetal and neithers.
Concept Introduction:
Hemiacetal is a type of molecule which contains carbon bonded to one -OH group and one -OR group. It is basically a half acetal. A hemiacetal forms when one molecule of alcohol reacts with a carbonyl group of an aldehyde or ketone. An acetal is the type of molecule where two −OR group is attached to a single carbon. An acetal forms when a hemi-acetal reacts with an alcohol.
For an Example:
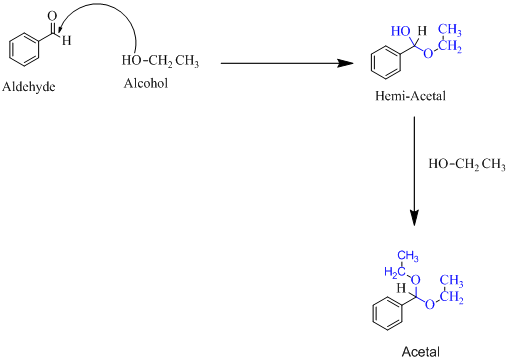
(c)
Interpretation:
To classify the given compounds as hemi-acetal, acetal and neithers.
Concept Introduction:
Hemiacetal is a type of molecule which contains carbon bonded to one -OH group and one -OR group. It is basically a half acetal. A hemiacetal forms when one molecule of alcohol reacts with a carbonyl group of an aldehyde or ketone. An acetal is the type of molecule where two −OR group is attached to a single carbon. An acetal forms when a hemi-acetal reacts with an alcohol.
For an Example:
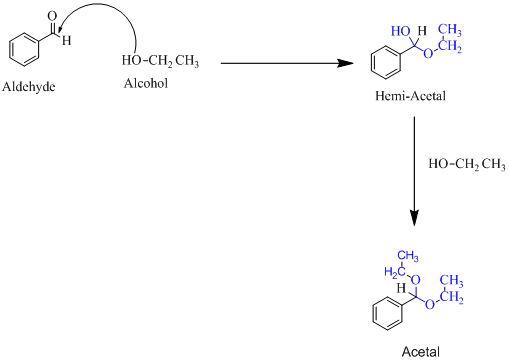
(d)
Interpretation:
To classify the given compounds as hemi-acetal, acetal and neithers.
Concept Introduction:
Hemiacetal is a type of molecule which contains carbon bonded to one -OH group and one -OR group. It is basically a half acetal. A hemiacetal forms when one molecule of alcohol reacts with a carbonyl group of an aldehyde or ketone. An acetal is the type of molecule where two −OR group is attached to a single carbon. An acetal forms when a hemi-acetal reacts with an alcohol.
For an Example:
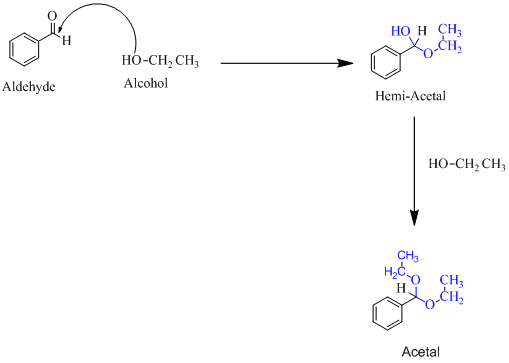
(e)
Interpretation:
To classify the given compounds as hemi-acetal, acetal and neithers.
Concept Introduction:
Hemiacetal is a type of molecule which contains carbon bonded to one -OH group and one -OR group. It is basically a half acetal. A hemiacetal forms when one molecule of alcohol reacts with a carbonyl group of an aldehyde or ketone. An acetal is the type of molecule where two −OR group is attached to a single carbon. An acetal forms when a hemi-acetal reacts with an alcohol.
For an Example:
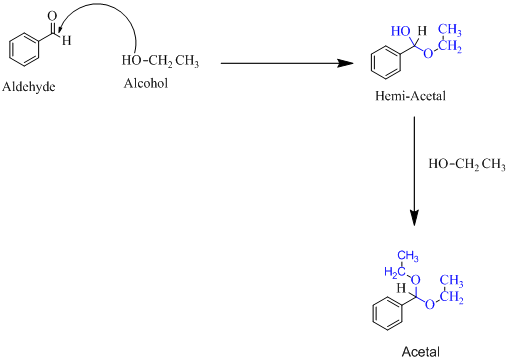
(f)
Interpretation:
To classify the given compounds as hemi-acetal, acetal and neithers.
Concept Introduction:
Hemiacetal is a type of molecule which contains carbon bonded to one -OH group and one -OR group. It is basically a half acetal. A hemiacetal forms when one molecule of alcohol reacts with a carbonyl group of an aldehyde or ketone. An acetal is the type of molecule where two −OR group is attached to a single carbon. An acetal forms when a hemi-acetal reacts with an alcohol.
For an Example:
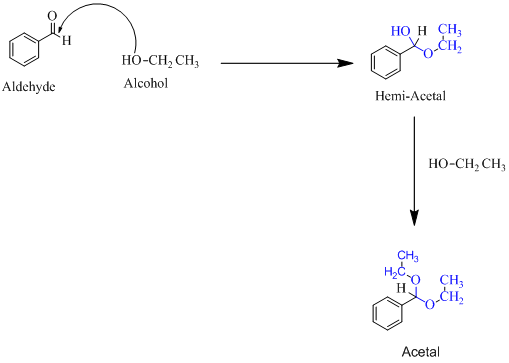
Trending nowThis is a popular solution!

Chapter 16 Solutions
Introduction To General, Organic, And Biochemistry
- Can you help me understand the CBC method on metal bridging by looking at this problem?arrow_forwardA partir de Aluminio y Co(NO3)2ꞏ6H2O, indicar las reacciones a realizar para obtener Azul de Thenard (Al2CoO4).arrow_forwardTo obtain Thenard Blue (Al2CoO4), the following reaction is correct (performed in an oven):Al(OH)3 + Co(OH)2 → Al2CoO4 + 4 H2Oarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning




