
Physics for Scientists and Engineers: Foundations and Connections
1st Edition
ISBN: 9781133939146
Author: Katz, Debora M.
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 59PQ
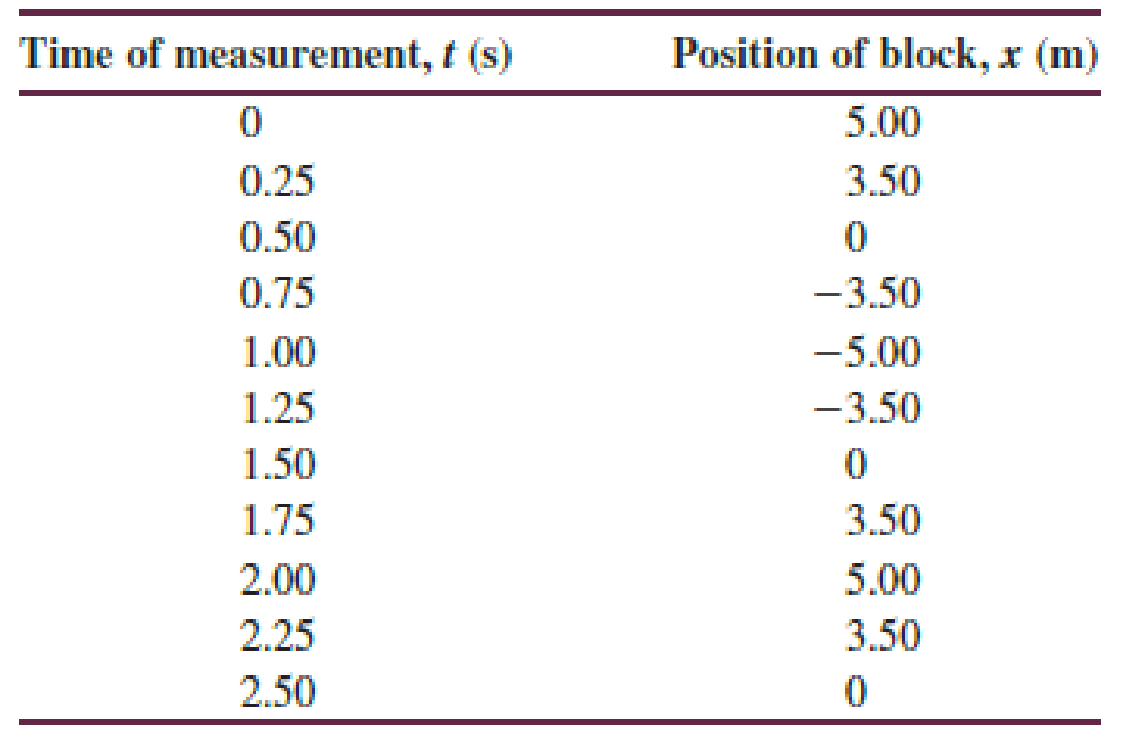
Table P16.59 gives the position of a block connected to a horizontal spring at several times. Sketch a motion diagram for the block.
Table P16.59
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
No chatgpt pls will upvote
Shown to the right is a block of mass m=5.71kgm=5.71kg on a ramp that makes an angle θ=24.1∘θ=24.1∘ with the horizontal. This block is being pushed by a horizontal force, F=229NF=229N. The coefficient of kinetic friction between the two surfaces is μ=0.51μ=0.51.
Enter an expression for the acceleration of the block up the ramp using variables from the problem statement together with gg for the acceleration due to gravity.
a=
If the density and atomic mass of copper are respectively 8.80 x 103 kg/m³ and 63.5 kg/kmol (note that 1 kmol = 1,000 mol), and copper has one free electron per copper atom, determine the following.
(a) the drift speed of the electrons in a 10 gauge copper wire (2.588 mm in diameter) carrying a 13.5 A current
1.988-4
See if you can obtain an expression for the drift speed of electrons in a copper wire in terms of the current in the wire, the diameter of the wire, the molecular weight and mass density of copper, Avogadro's number, and the charge
on an electron. m/s
(b) the Hall voltage if a 2.68 T field is applied perpendicular to the wire
3.34e-6
x
Can you start with basic equations for the electric and magnetic forces acting on the electrons moving through the wire and obtain a relationship between the magnitude of the electric and magnetic field and the drift speed of the
electrons? How is the magnitude of the electric field related to the Hall voltage and the diameter of the wire? V
Chapter 16 Solutions
Physics for Scientists and Engineers: Foundations and Connections
Ch. 16.1 - Prob. 16.1CECh. 16.2 - Prob. 16.2CECh. 16.2 - For each expression, identify the angular...Ch. 16.5 - Prob. 16.4CECh. 16.6 - Prob. 16.5CECh. 16.6 - Prob. 16.6CECh. 16 - Case Study For each velocity listed, state the...Ch. 16 - Case Study For each acceleration listed, state the...Ch. 16 - Prob. 3PQCh. 16 - Prob. 4PQ
Ch. 16 - Prob. 5PQCh. 16 - Prob. 6PQCh. 16 - The equation of motion of a simple harmonic...Ch. 16 - The expression x = 8.50 cos (2.40 t + /2)...Ch. 16 - A simple harmonic oscillator has amplitude A and...Ch. 16 - Prob. 10PQCh. 16 - A 1.50-kg mass is attached to a spring with spring...Ch. 16 - Prob. 12PQCh. 16 - Prob. 13PQCh. 16 - When the Earth passes a planet such as Mars, the...Ch. 16 - A point on the edge of a childs pinwheel is in...Ch. 16 - Prob. 16PQCh. 16 - Prob. 17PQCh. 16 - A jack-in-the-box undergoes simple harmonic motion...Ch. 16 - C, N A uniform plank of length L and mass M is...Ch. 16 - Prob. 20PQCh. 16 - A block of mass m = 5.94 kg is attached to a...Ch. 16 - A block of mass m rests on a frictionless,...Ch. 16 - It is important for astronauts in space to monitor...Ch. 16 - Prob. 24PQCh. 16 - A spring of mass ms and spring constant k is...Ch. 16 - In an undergraduate physics lab, a simple pendulum...Ch. 16 - A simple pendulum of length L hangs from the...Ch. 16 - We do not need the analogy in Equation 16.30 to...Ch. 16 - Prob. 29PQCh. 16 - Prob. 30PQCh. 16 - Prob. 31PQCh. 16 - Prob. 32PQCh. 16 - Prob. 33PQCh. 16 - Show that angular frequency of a physical pendulum...Ch. 16 - A uniform annular ring of mass m and inner and...Ch. 16 - A child works on a project in art class and uses...Ch. 16 - Prob. 37PQCh. 16 - Prob. 38PQCh. 16 - In the short story The Pit and the Pendulum by...Ch. 16 - Prob. 40PQCh. 16 - A restaurant manager has decorated his retro diner...Ch. 16 - Prob. 42PQCh. 16 - A wooden block (m = 0.600 kg) is connected to a...Ch. 16 - Prob. 44PQCh. 16 - Prob. 45PQCh. 16 - Prob. 46PQCh. 16 - Prob. 47PQCh. 16 - Prob. 48PQCh. 16 - A car of mass 2.00 103 kg is lowered by 1.50 cm...Ch. 16 - Prob. 50PQCh. 16 - Prob. 51PQCh. 16 - Prob. 52PQCh. 16 - Prob. 53PQCh. 16 - Prob. 54PQCh. 16 - Prob. 55PQCh. 16 - Prob. 56PQCh. 16 - Prob. 57PQCh. 16 - An ideal simple harmonic oscillator comprises a...Ch. 16 - Table P16.59 gives the position of a block...Ch. 16 - Use the position data for the block given in Table...Ch. 16 - Consider the position data for the block given in...Ch. 16 - Prob. 62PQCh. 16 - Prob. 63PQCh. 16 - Use the data in Table P16.59 for a block of mass m...Ch. 16 - Consider the data for a block of mass m = 0.250 kg...Ch. 16 - A mass on a spring undergoing simple harmonic...Ch. 16 - A particle initially located at the origin...Ch. 16 - Consider the system shown in Figure P16.68 as...Ch. 16 - Prob. 69PQCh. 16 - Prob. 70PQCh. 16 - Prob. 71PQCh. 16 - Prob. 72PQCh. 16 - Determine the period of oscillation of a simple...Ch. 16 - The total energy of a simple harmonic oscillator...Ch. 16 - A spherical bob of mass m and radius R is...Ch. 16 - Prob. 76PQCh. 16 - A lightweight spring with spring constant k = 225...Ch. 16 - Determine the angular frequency of oscillation of...Ch. 16 - Prob. 79PQCh. 16 - A Two springs, with spring constants k1 and k2,...Ch. 16 - Prob. 81PQCh. 16 - Prob. 82PQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- (a) At what speed (in m/s) will a proton move in a circular path of the same radius as an electron that travels at 7.85 x 100 m/s perpendicular to the Earth's magnetic field at an altitude where the field strength is 1.20 x 10-5 T? 4.27e3 m/s (b) What would the radius (in m) of the path be if the proton had the same speed as the electron? 0.685 x m (c) What would the radius (in m) be if the proton had the same kinetic energy as the electron? 0.0084 m (d) What would the radius (in m) be if the proton had the same momentum as the electron? 0.0303 x marrow_forwardTwo charges are placed on the x axis. One of the charges (91 = +6.63 μC) is at x₁ = +3.00 cm and the other (92 = -24.2 μC) is at x2 = +9.00 cm. Find the net electric field (magnitude and direction given as a plus or minus sign) at (a) x = 0 cm and (b) x = +6.00 cm.arrow_forwardThe diagram shows the all of the forces acting on a body of mass 2.76 kg. The three forces have magnitudes F1 = 65.2 N, F2 = 21.6 N, and F3 = 77.9 N, with directions as indicted in the diagram, where θ = 49.9 degrees and φ = 21.1 degrees. The dashed lines are parallel to the x and y axes. At t = 0, the body is moving at a speed of 6.87 m/s in the positive x direction. a. whats the x component of the acceleration? b. whats the y component of the acceleration? c. whats the speed of the body in m/s at t = 12.3s? d. whats the magnitude of the displacement of the body n meters between t = 0 and 12.3s?arrow_forward
- No chatgpt pls will upvotearrow_forwardNo chatgpt pls will upvotearrow_forwardA cylinder with a piston contains 0.153 mol of nitrogen at a pressure of 1.83×105 Pa and a temperature of 290 K. The nitrogen may be treated as an ideal gas. The gas is first compressed isobarically to half its original volume. It then expands adiabatically back to its original volume, and finally it is heated isochorically to its original pressure. Part A Compute the temperature at the beginning of the adiabatic expansion. Express your answer in kelvins. ΕΠΙ ΑΣΦ T₁ = ? K Submit Request Answer Part B Compute the temperature at the end of the adiabatic expansion. Express your answer in kelvins. Π ΑΣΦ T₂ = Submit Request Answer Part C Compute the minimum pressure. Express your answer in pascals. ΕΠΙ ΑΣΦ P = Submit Request Answer ? ? K Paarrow_forward
- Learning Goal: To understand the meaning and the basic applications of pV diagrams for an ideal gas. As you know, the parameters of an ideal gas are described by the equation pV = nRT, where p is the pressure of the gas, V is the volume of the gas, n is the number of moles, R is the universal gas constant, and T is the absolute temperature of the gas. It follows that, for a portion of an ideal gas, pV = constant. Τ One can see that, if the amount of gas remains constant, it is impossible to change just one parameter of the gas: At least one more parameter would also change. For instance, if the pressure of the gas is changed, we can be sure that either the volume or the temperature of the gas (or, maybe, both!) would also change. To explore these changes, it is often convenient to draw a graph showing one parameter as a function of the other. Although there are many choices of axes, the most common one is a plot of pressure as a function of volume: a pV diagram. In this problem, you…arrow_forwardLearning Goal: To understand the meaning and the basic applications of pV diagrams for an ideal gas. As you know, the parameters of an ideal gas are described by the equation pV = nRT, where p is the pressure of the gas, V is the volume of the gas, n is the number of moles, R is the universal gas constant, and T is the absolute temperature of the gas. It follows that, for a portion of an ideal gas, pV = constant. T One can see that, if the amount of gas remains constant, it is impossible to change just one parameter of the gas: At least one more parameter would also change. For instance, if the pressure of the gas is changed, we can be sure that either the volume or the temperature of the gas (or, maybe, both!) would also change. To explore these changes, it is often convenient to draw a graph showing one parameter as a function of the other. Although there are many choices of axes, the most common one is a plot of pressure as a function of volume: a pV diagram. In this problem, you…arrow_forward■ Review | Constants A cylinder with a movable piston contains 3.75 mol of N2 gas (assumed to behave like an ideal gas). Part A The N2 is heated at constant volume until 1553 J of heat have been added. Calculate the change in temperature. ΜΕ ΑΣΦ AT = Submit Request Answer Part B ? K Suppose the same amount of heat is added to the N2, but this time the gas is allowed to expand while remaining at constant pressure. Calculate the temperature change. AT = Π ΑΣΦ Submit Request Answer Provide Feedback ? K Nextarrow_forward
- 4. I've assembled the following assortment of point charges (-4 μC, +6 μC, and +3 μC) into a rectangle, bringing them together from an initial situation where they were all an infinite distance away from each other. Find the electric potential at point "A" (marked by the X) and tell me how much work it would require to bring a +10.0 μC charge to point A if it started an infinite distance away (assume that the other three charges remains fixed). 300 mm -4 UC "A" 0.400 mm +6 UC +3 UC 5. It's Friday night, and you've got big party plans. What will you do? Why, make a capacitor, of course! You use aluminum foil as the plates, and since a standard roll of aluminum foil is 30.5 cm wide you make the plates of your capacitor each 30.5 cm by 30.5 cm. You separate the plates with regular paper, which has a thickness of 0.125 mm and a dielectric constant of 3.7. What is the capacitance of your capacitor? If you connect it to a 12 V battery, how much charge is stored on either plate? =arrow_forwardLearning Goal: To understand the meaning and the basic applications of pV diagrams for an ideal gas. As you know, the parameters of an ideal gas are described by the equation pV = nRT, where p is the pressure of the gas, V is the volume of the gas, n is the number of moles, R is the universal gas constant, and T is the absolute temperature of the gas. It follows that, for a portion of an ideal gas, PV T = constant. One can see that, if the amount of gas remains constant, it is impossible to change just one parameter of the gas: At least one more parameter would also change. For instance, if the pressure of the gas is changed, we can be sure that either the volume or the temperature of the gas (or, maybe, both!) would also change. To explore these changes, it is often convenient to draw a graph showing one parameter as a function of the other. Although there are many choices of axes, the most common one is a plot of pressure as a function of volume: a pV diagram. In this problem, you…arrow_forwardA-e pleasearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers with Modern ...PhysicsISBN:9781337553292Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781285737027Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University
University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers with Modern ...
Physics
ISBN:9781337553292
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

College Physics
Physics
ISBN:9781285737027
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics Volume 1
Physics
ISBN:9781938168277
Author:William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:OpenStax - Rice University
SIMPLE HARMONIC MOTION (Physics Animation); Author: EarthPen;https://www.youtube.com/watch?v=XjkUcJkGd3Y;License: Standard YouTube License, CC-BY