
(a)
Interpretation:
IUPAC name for the acrylic acid has to be given.
Concept Introduction:
For naming a
IUPAC rules for naming a carboxylic acid:
- The longest parent carbon chain is identified that includes the carboxyl group.
- The parent chain name is changed by replacing the suffix “-e” with “-oic acid”.
- Numbering is done in a way that the carboxyl group is designated as number 1. This is not indicated in the part of the name because for
aldehyde , the carboxyl carbon is always numbered 1. - The identity and location of substituents if any has to be determined and this information has to be added in front of the IUPAC name.
- If the carboxyl
functional group is attached to a ring of carbon atoms, the ring is named and “-carboxylic acid” is added as suffix. - If the compound contains two carboxyl groups, then suffix “-dioic acid” is added after the parent alkane name.
(a)
Answer to Problem 16.33EP
IUPAC name of acrylic acid is propenoic acid.
Explanation of Solution
Structure of acrylic acid is,
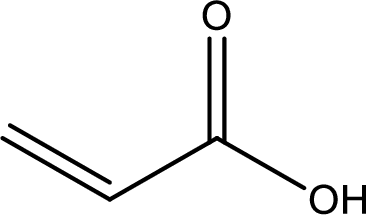
The longest continuous carbon chain has to be found out with the carboxyl group in it. In this it is a three carbon chain. The structure contains a double bond between carbon atoms. Hence, the parent is propene. The given structure contains a carboxyl group. The carboxylic acid is named by replacing the suffix “-e” with suffix “-oic acid”. This gives the name of carboxylic acid as propenoic acid.
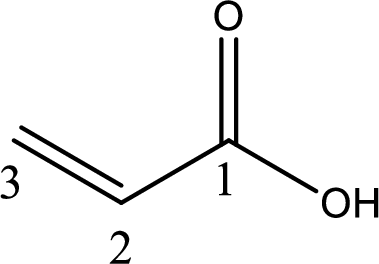
Looking for substituents it is found that there are no substituents present on the carbon chain. Hence, the IUPAC name of the acrylic acid is propenoic acid.
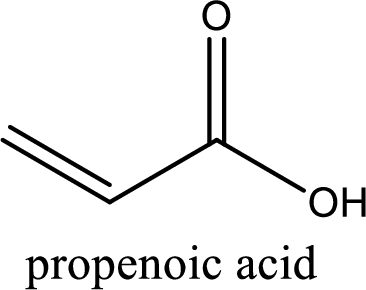
IUPAC name of acrylic acid is given.
(b)
Interpretation:
IUPAC name for the lactic acid has to be given.
Concept Introduction:
For naming a carboxylic acid in IUPAC nomenclature, the suffix “-oic” is added to the parent alkane name.
IUPAC rules for naming a carboxylic acid:
- The longest parent carbon chain is identified that includes the carboxyl group.
- The parent chain name is changed by replacing the suffix “-e” with “-oic acid”.
- Numbering is done in a way that the carboxyl group is designated as number 1. This is not indicated in the part of the name because for aldehyde, the carboxyl carbon is always numbered 1.
- The identity and location of substituents if any has to be determined and this information has to be added in front of the IUPAC name.
- If the carboxyl functional group is attached to a ring of carbon atoms, the ring is named and “-carboxylic acid” is added as suffix.
- If the compound contains two carboxyl groups, then suffix “-dioic acid” is added after the parent alkane name.
(b)
Answer to Problem 16.33EP
IUPAC name of lactic acid is 2-hydroxypropanoic acid.
Explanation of Solution
Structure of lactic acid is,
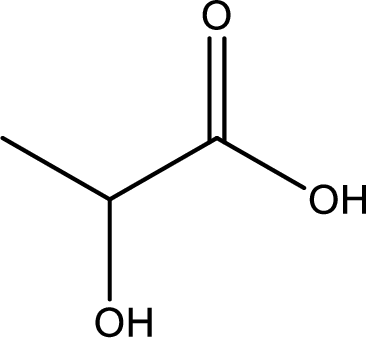
The longest continuous carbon chain has to be found out with the carboxyl group in it. In this it is a three carbon chain. The parent alkane is propane. The given structure contains a carboxyl group. The carboxylic acid is named by replacing the suffix “-e” with suffix “-oic acid”. This gives the name of carboxylic acid as propanoic acid.
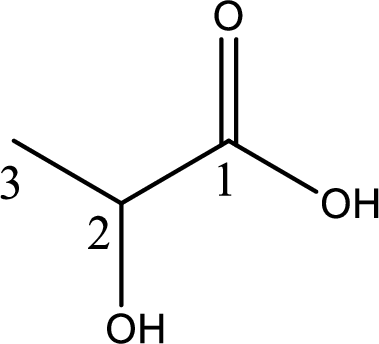
Looking for substituents it is found that there is a hydroxyl group at the second carbon atom. Hence, the IUPAC name of the lactic acid is 2-hydroxypropanoic acid.
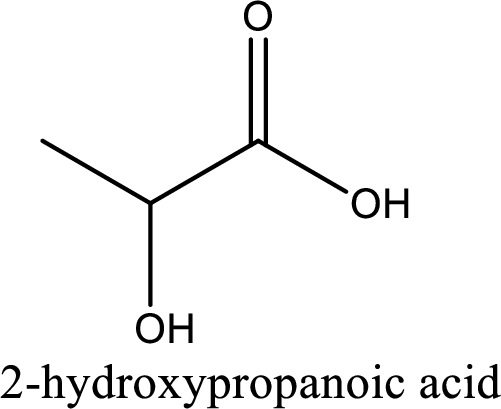
IUPAC name of lactic acid is given.
(c)
Interpretation:
IUPAC name for the maleic acid has to be given.
Concept Introduction:
For naming a carboxylic acid in IUPAC nomenclature, the suffix “-oic” is added to the parent alkane name.
IUPAC rules for naming a carboxylic acid:
- The longest parent carbon chain is identified that includes the carboxyl group.
- The parent chain name is changed by replacing the suffix “-e” with “-oic acid”.
- Numbering is done in a way that the carboxyl group is designated as number 1. This is not indicated in the part of the name because for aldehyde, the carboxyl carbon is always numbered 1.
- The identity and location of substituents if any has to be determined and this information has to be added in front of the IUPAC name.
- If the carboxyl functional group is attached to a ring of carbon atoms, the ring is named and “-carboxylic acid” is added as suffix.
- If the compound contains two carboxyl groups, then suffix “-dioic acid” is added after the parent alkane name.
(c)
Answer to Problem 16.33EP
IUPAC name of maleic acid is cis-butenedioic acid.
Explanation of Solution
Structure of maleic acid is,
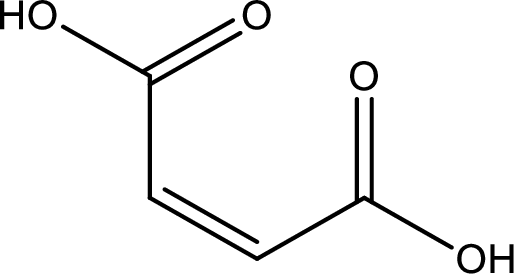
The longest continuous carbon chain has to be found out with the carboxyl group in it. In this it is a four carbon chain. The structure contains a double bond in it. The parent carbon chain is butene. The given structure contains two carboxyl groups. The carboxylic acid is named by adding suffix “-dioic acid”. This gives the name of carboxylic acid as butenedioic acid.
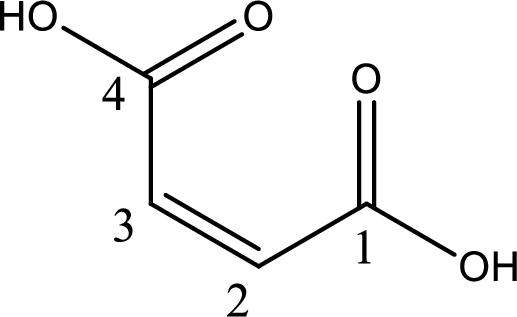
Looking for substituents it is found that there are no substituents present in the carbon chain. Stereochemistry is possible across the double bond. As the two hydrogen atoms are on the same side of double bond, the configuration at the double bond is “cis”. This has to be included in the name to get the IUPAC name. IUPAC name of the maleic acid is found as cis-butenedioic acid.
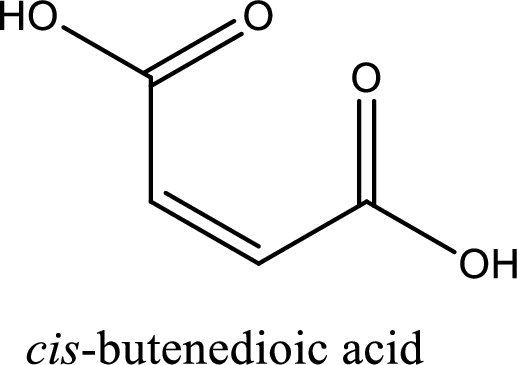
IUPAC name of maleic acid is given.
(d)
Interpretation:
IUPAC name for the glycolic acid has to be given.
Concept Introduction:
For naming a carboxylic acid in IUPAC nomenclature, the suffix “-oic” is added to the parent alkane name.
IUPAC rules for naming a carboxylic acid:
- The longest parent carbon chain is identified that includes the carboxyl group.
- The parent chain name is changed by replacing the suffix “-e” with “-oic acid”.
- Numbering is done in a way that the carboxyl group is designated as number 1. This is not indicated in the part of the name because for aldehyde, the carboxyl carbon is always numbered 1.
- The identity and location of substituents if any has to be determined and this information has to be added in front of the IUPAC name.
- If the carboxyl functional group is attached to a ring of carbon atoms, the ring is named and “-carboxylic acid” is added as suffix.
- If the compound contains two carboxyl groups, then suffix “-dioic acid” is added after the parent alkane name.
(d)
Answer to Problem 16.33EP
IUPAC name of glycolic acid is 2-hydroxyethanoic acid.
Explanation of Solution
Structure of glycolic acid is,
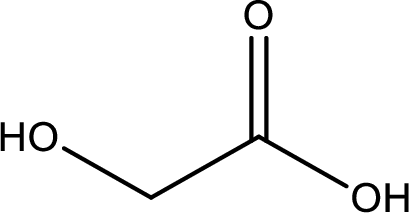
The longest continuous carbon chain has to be found out with the carboxyl group in it. In this it is a two carbon chain. The parent alkane is ethane. The given structure contains a carboxyl group. The carboxylic acid is named by replacing the suffix “-e” with suffix “-oic acid”. This gives the name of carboxylic acid as ethanoic acid.
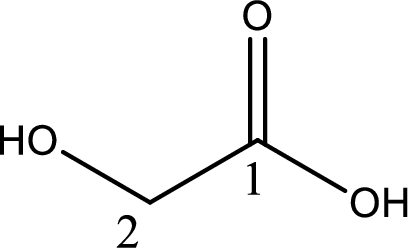
Looking for substituents it is found that there is a hydroxyl group at the second carbon atom. Hence, the IUPAC name of the glycolic acid is 2-hydroxyethanoic acid.
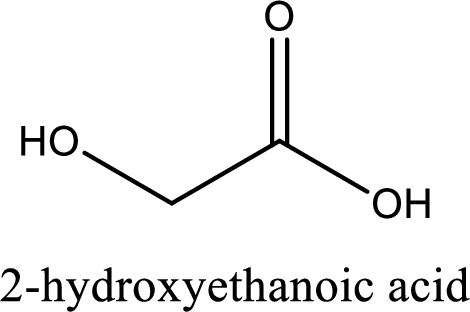
IUPAC name of glycolic acid is given.
Want to see more full solutions like this?
Chapter 16 Solutions
GENERAL,ORGANIC,+BIO.CHEM.-MINDTAP
- Identify and provide a concise explanation of a specific analytical instrument capable of detecting and quantifying trace compounds in food samples. Emphasise the instrumental capabilities relevant to trace compound analysis in the nominated food. Include the specific application name (eg: identification and quantification of mercury in salmon), outline a brief description of sample preparation procedures, and provide a summary of the obtained results from the analytical process.arrow_forwardIdentify and provide an explanation of what 'Seperation Science' is. Also describe its importance with the respect to the chemical analysis of food. Provide specific examples.arrow_forward5. Propose a Synthesis for the molecule below. You may use any starting materials containing 6 carbons or less (reagents that aren't incorporated into the final molecule such as PhзP do not count towards this total, and the starting material can have whatever non-carbon functional groups you want), and any of the reactions you have learned so far in organic chemistry I, II, and III. Your final answer should show each step separately, with intermediates and conditions clearly drawn. H3C CH3arrow_forward
- State the name and condensed formula of isooxazole obtained by reacting acetylacetone and hydroxylamine.arrow_forwardState the name and condensed formula of the isothiazole obtained by reacting acetylacetone and thiosemicarbazide.arrow_forwardProvide the semi-developed formula of isooxazole obtained by reacting acetylacetone and hydroxylamine.arrow_forward
- Given a 1,3-dicarbonyl compound (R1-CO-CH2-CO-R2), indicate the formula of the compound obtaineda) if I add hydroxylamine (NH2OH) to give an isooxazole.b) if I add thiosemicarbazide (NH2-CO-NH-NH2) to give an isothiazole.arrow_forwardAn orange laser has a wavelength of 610 nm. What is the energy of this light?arrow_forwardThe molar absorptivity of a protein in water at 280 nm can be estimated within ~5-10% from its content of the amino acids tyrosine and tryptophan and from the number of disulfide linkages (R-S-S-R) between cysteine residues: Ε280 nm (M-1 cm-1) ≈ 5500 nTrp + 1490 nTyr + 125 nS-S where nTrp is the number of tryptophans, nTyr is the number of tyrosines, and nS-S is the number of disulfide linkages. The protein human serum transferrin has 678 amino acids including 8 tryptophans, 26 tyrosines, and 19 disulfide linkages. The molecular mass of the most dominant for is 79550. Predict the molar absorptivity of transferrin. Predict the absorbance of a solution that’s 1.000 g/L transferrin in a 1.000-cm-pathlength cuvet. Estimate the g/L of a transferrin solution with an absorbance of 1.50 at 280 nm.arrow_forward
- In GC, what order will the following molecules elute from the column? CH3OCH3, CH3CH2OH, C3H8, C4H10arrow_forwardBeer’s Law is A = εbc, where A is absorbance, ε is the molar absorptivity (which is specific to the compound and wavelength in the measurement), and c is concentration. The absorbance of a 2.31 × 10-5 M solution of a compound is 0.822 at a wavelength of 266 nm in a 1.00-cm cell. Calculate the molar absorptivity at 266 nm.arrow_forwardHow to calculate % of unknown solution using line of best fit y=0.1227x + 0.0292 (y=2.244)arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





