
Concept explainers
(a)
Interpretation:
Among the given designations, the one or more which may be correct for the given situation has to be chosen.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom.
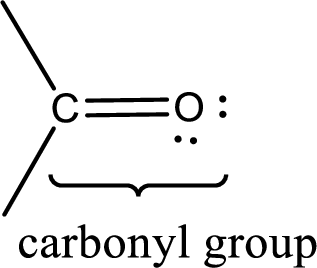
If a hydroxyl group is attached to a carbonyl group means it is known as carboxyl group. This can be represented as shown below,
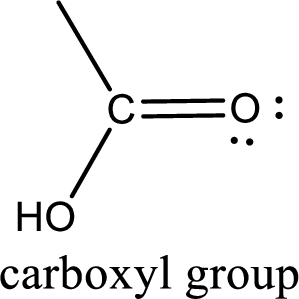
Carbon bonded to an oxygen atom through double bond is known as carbonyl group. The carbonyl group bonded to a R group means it is known as acyl group. If the acyl group is bonded to oxygen, nitrogen or halogen atom then the compound is known as acyl compound. If the acyl group is bonded to a carbon or hydrogen atom, then the compound is known as carbonyl compound. If the carbonyl carbon atom is bonded to a hydroxyl group then it is a carboxylic acid.
(b)
Interpretation:
Among the given designations, the one or more which may be correct for the given situation has to be chosen.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom. Aldehydes and ketones possess this carbonyl functional group in it. The structural representation of a carbonyl group can be given as shown below,
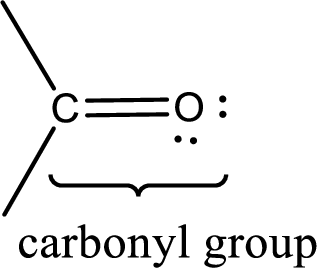
If a hydroxyl group is attached to a carbonyl group means it is known as carboxyl group. This can be represented as shown below,
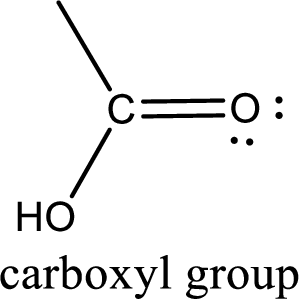
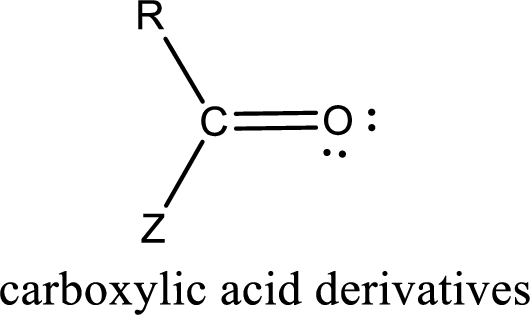
Carboxylic acid derivatives are the ones that are synthesized from or converted to a carboxylic acid. The generalized structural representation of carboxylic acid derivatives is shown below,
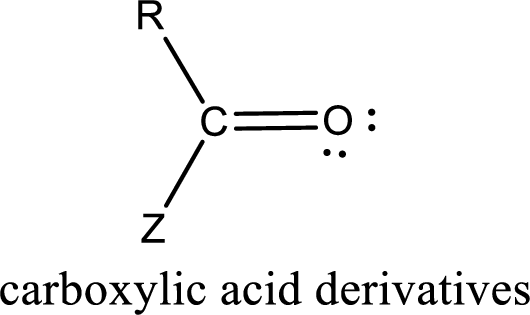
Carbon bonded to an oxygen atom through double bond is known as carbonyl group. The carbonyl group bonded to a R group means it is known as acyl group. If the acyl group is bonded to oxygen, nitrogen or halogen atom then the compound is known as acyl compound. If the acyl group is bonded to a carbon or hydrogen atom, then the compound is known as carbonyl compound. If the carbonyl carbon atom is bonded to a hydroxyl group then it is a carboxylic acid.
(c)
Interpretation:
Among the given designations, the one or more which may be correct for the given situation has to be chosen.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom. Aldehydes and ketones possess this carbonyl functional group in it. The structural representation of a carbonyl group can be given as shown below,
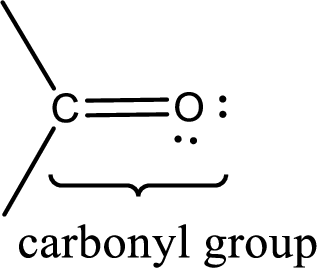
If a hydroxyl group is attached to a carbonyl group means it is known as carboxyl group. This can be represented as shown below,
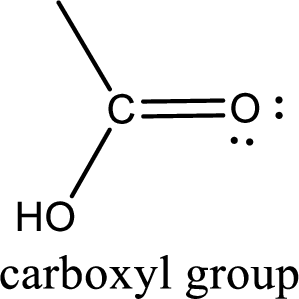
Carboxylic acid derivatives are the ones that are synthesized from or converted to a carboxylic acid. The generalized structural representation of carboxylic acid derivatives is shown below,
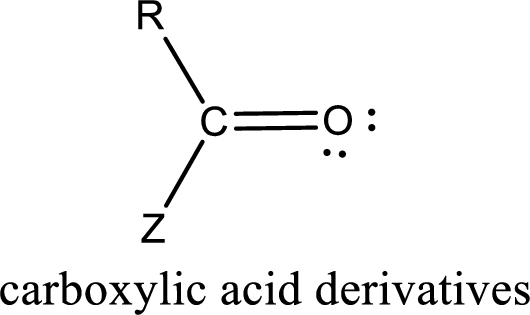
Carbon bonded to an oxygen atom through double bond is known as carbonyl group. The carbonyl group bonded to a R group means it is known as acyl group. If the acyl group is bonded to oxygen, nitrogen or halogen atom then the compound is known as acyl compound. If the acyl group is bonded to a carbon or hydrogen atom, then the compound is known as carbonyl compound. If the carbonyl carbon atom is bonded to a hydroxyl group then it is a carboxylic acid.
(d)
Interpretation:
Among the given designations, the one or more which may be correct for the given situation has to be chosen.
Concept Introduction:
Carbonyl groups are the one which contain a double bond between carbon and oxygen atom. Aldehydes and ketones possess this carbonyl functional group in it. The structural representation of a carbonyl group can be given as shown below,
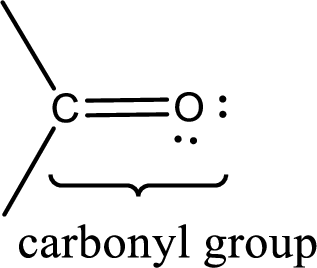
If a hydroxyl group is attached to a carbonyl group means it is known as carboxyl group. This can be represented as shown below,
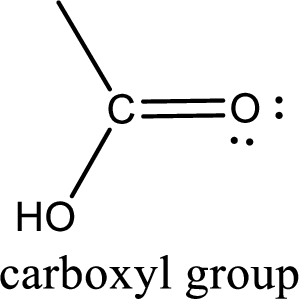
Carboxylic acid derivatives are the ones that are synthesized from or converted to a carboxylic acid. The generalized structural representation of carboxylic acid derivatives is shown below,
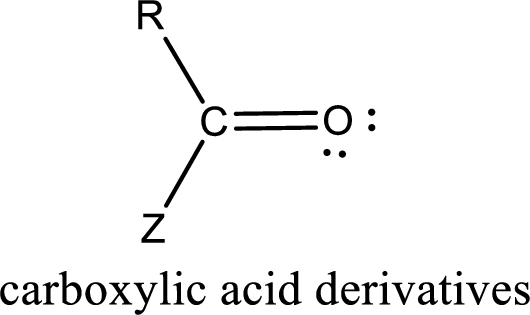
Carbon bonded to an oxygen atom through double bond is known as carbonyl group. The carbonyl group bonded to a R group means it is known as acyl group. If the acyl group is bonded to oxygen, nitrogen or halogen atom then the compound is known as acyl compound. If the acyl group is bonded to a carbon or hydrogen atom, then the compound is known as carbonyl compound. If the carbonyl carbon atom is bonded to a hydroxyl group then it is a carboxylic acid.
Want to see the full answer?
Check out a sample textbook solution
Chapter 16 Solutions
GENERAL,ORGANIC,+BIO.CHEM.-MINDTAP
- Predict the products of this organic reaction: + H₂O H* ? A Specifically, in the drawing area below draw the skeletal ("line") structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click and drag to start drawing a structure.arrow_forwardPredict the major organic products of the reaction below and draw them on right side of the arrow. If there will be no significant reaction, check the box below the drawing area instead. C Cl CH, OH There will be no significant reaction. + pyridine G Click and drag to start drawing a structure.arrow_forwardWhat is the missing reactant in this organic reaction? H R+ H2O Δ OH 0= CH3-CH-O-CH3 + CH3-C-OH Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. No Answer Click anywhere to draw the first atom of your structure. dyarrow_forward
- You are trying to determine whether the following organic reaction can be done in a single synthesis step. If so, add any missing reagents or conditions in the drawing area below. If it isn't possible to do this reaction in a single synthesis step, check the box below the drawing area instead. Note for advanced students: if you have a choice of reagents to add, you should choose the least reactive and most economical reagents possible. Cl It isn't possible to do this reaction in a single synthesis step. + T OHarrow_forwardPredict the products of this organic reaction: CH3 O CH3-CH-C-O-CH2-CH2-CH3 + H₂OH+ Η ? A Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. No Reaction Click anywhere to draw the first atom of your structure.arrow_forward€ CH3-CH-C-O-CH2-CH2-CH3 + NaOH A? Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. (If there's more than one product, draw them in any arrangement you like, so long as they aren't touching.) If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. Predict the products of this organic reaction: CH3 O Click anywhere to draw the first atom of your structure. No reaction ✓ Garrow_forward
- A molecule can have a temporary or permanent depending on the structure and the way the electrons can move. True Falsearrow_forwardedict the products of this organic reaction: CH3 O A CH3-CH-C-NH2 + H2O + HCI ? Specifically, in the drawing area below draw the condensed structure of the product, or products, of this reaction. If there's more than one product, draw them in any arrangement you like, so long as they aren't touching. If there aren't any products because this reaction won't happen, check the No reaction box under the drawing area. Click anywhere to draw the first atom of your structure. No Reaction planation Check 2025 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center +arrow_forwardDraw the mechanism for the following reaction: OH A few notes: CI O • You may assume that each reagent is present in whatever amount you need to draw your mechanism. • To save you some time, one of the starting materials has been copied into the first step of the drawing area. AP Add/Remove step Cl Click and drag to start drawing a structure.arrow_forward
- What is the missing reactant in this organic reaction? CH3 O [0] R CH3-CH-C-OH Specifically, in the drawing area below draw the condensed structure of R. If there is more than one reasonable answer, you can draw any one of them. If there is no reasonable answer, check the No answer box under the drawing area. Note: the organic equation above only shows the important organic reactant and product. Minor small-molecule reactants or products (like H₂O) are not shown. No Answer Click anywhere to draw the first atom of your structure. ×arrow_forwardPredict the product of this organic reaction: O CH3 A NH3 + HO–C—CH—CH, P+H₂O Specifically, in the drawing area below draw the condensed structure of P. If there is no reasonable possibility for P, check the No answer box under the drawing area. No Answer Click anywhere to draw the first atom of your structure. A 5arrow_forwardFor a reaction to be spontaneous, the Gibbs Free Energy must be less than zero. True or Falsearrow_forward
- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning 
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning





