
(a)
Interpretation:
The structural formula for the product of hydrolysis of given acetal in aqueous HCl has to be drawn.
Concept Introduction:
The structural formula of a compound shows how the atoms are arranged in a molecule and, in particular, shows which
Hydrocarbon compound which contain a hydroxyl group and an alkoxy group is known as Hemiacetals. Hemiacetal can be prepared by the addition of alcohol to an
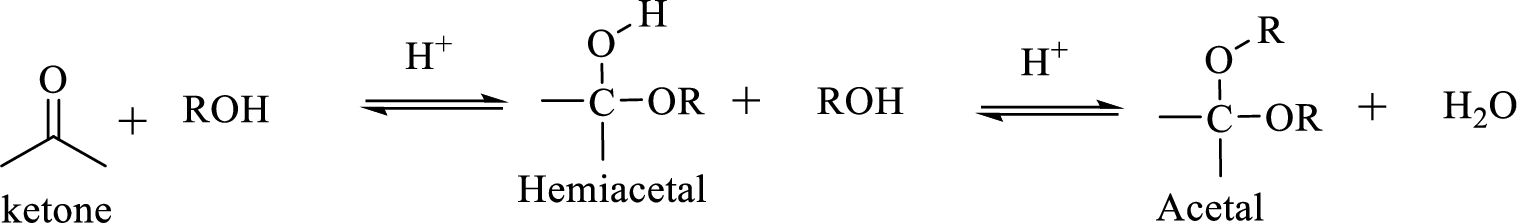
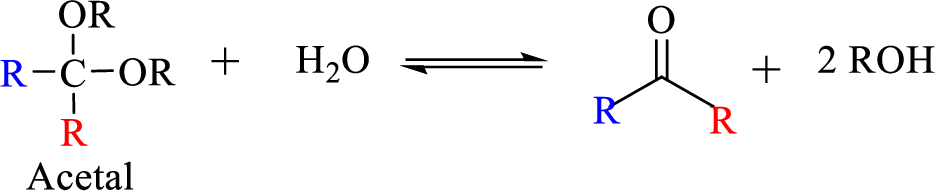
The process of hydrolysis of acetal exactly the reverse of acetal formation and in this reaction hemiacetal acts as the intermediate. The reaction is shown below,
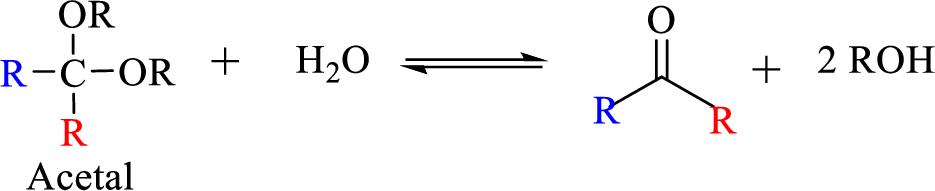
(b)
Interpretation:
The structural formula for the product of hydrolysis of given acetal in aqueous HCl has to be drawn.
Concept Introduction:
The structural formula of a compound shows how the atoms are arranged in a molecule and, in particular, shows which functional groups are present.
Hydrocarbon compound which contain a hydroxyl group and an alkoxy group is known as Hemiacetals. Hemiacetal can be prepared by the addition of alcohol to an aldehyde or ketone in the presence of an acid catalyst. Hemiacetals react with a second alcohol molecule to produce an acetal, which contains a carbon with two alkoxy groups –OR.
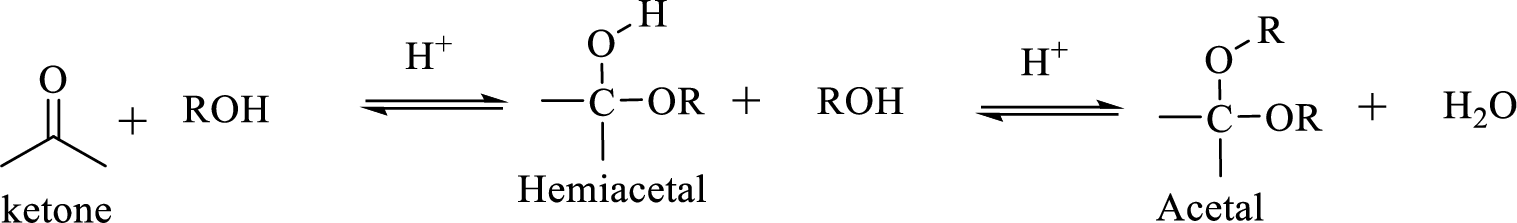
The process of hydrolysis of acetal exactly the reverse of acetal formation and in this reaction hemiacetal acts as the intermediate. The reaction is shown below,
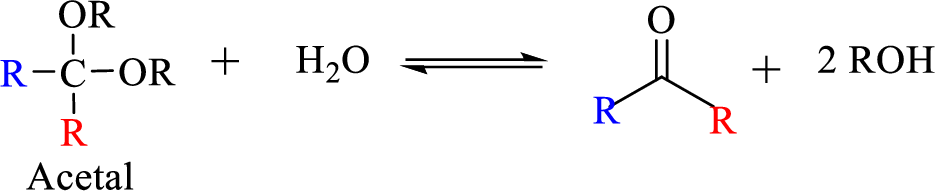
(c)
Interpretation:
The structural formula for the product of hydrolysis of given acetal in aqueous HCl has to be drawn.
Concept Introduction:
The structural formula of a compound shows how the atoms are arranged in a molecule and, in particular, shows which functional groups are present.
Hydrocarbon compound which contain a hydroxyl group and an alkoxy group is known as Hemiacetals. Hemiacetal can be prepared by the addition of alcohol to an aldehyde or ketone in the presence of an acid catalyst. Hemiacetals react with a second alcohol molecule to produce an acetal, which contains a carbon with two alkoxy groups –OR.
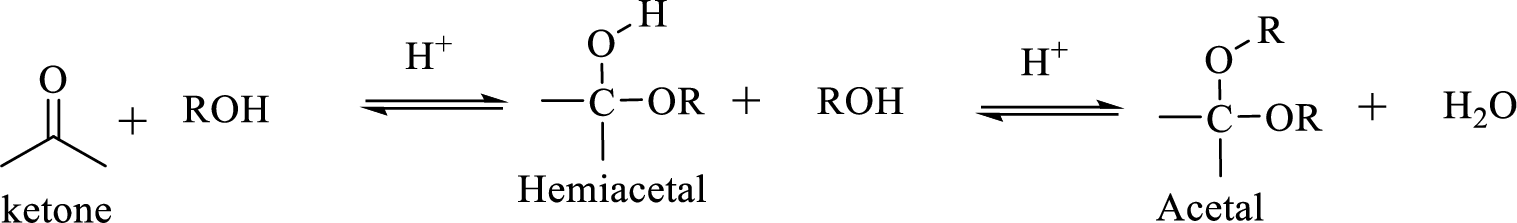
The process of hydrolysis of acetal exactly the reverse of acetal formation and in this reaction hemiacetal acts as the intermediate. The reaction is shown below,
Trending nowThis is a popular solution!

Chapter 16 Solutions
Organic Chemistry
- -C = C - C - + Br₂ + I" -> -C-C-c -C = C -C- + Br² + I₂ -C=C Br I + Brū + Iz -7- C - C-C- I Br Mechanism; - C = c - c - + Br - Br > - C-c-c- Br -C-C-C- + 1 - - -Ċ-Ċ'-c' - Br Br Iarrow_forwardWrite the mechanism of the esterification reaction (please show the mechanism included line pairs and arrows)arrow_forwardHow do I break down the reaction shown on the chalkboard and explain it correctly using the bromonium ion mechanism, instead of the (disproven) carbocation-based mechanismarrow_forward
- ¿Qué the product is obtained from tetraethoxypropano and hidrazina?. Indicate the reason why the corresponding dial is used.arrow_forwardIf CH3COCH2CH(OCH3)2 is reacted with hydrazine, two isomeric products are formed. Indicate their structures and the major product.arrow_forwardIs it possible to obtain addition derivatives to nitrogen in position 2 of pyrazoles by reaction with electrophilic agents? Reason for this.arrow_forward
- Starting from 1,3-dicarbonyl derivatives to obtain isooxazoles and isothiazoles. Indicate whether synthetic methods exist.arrow_forwardIn the synthesis of benzotriazole, adding NaNO2 heats the solution. State the reason.arrow_forwardIndicate the products obtained by treating benzotriazole with dimethyl sulfate or methyl iodide in a basic medium.arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning




