
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 16, Problem 16.67P
Treatment of β-D-glucose with methanol in the presence of an acid catalyst converts it into a mixture of two compounds called methyl glucosides (Section 25.3A).
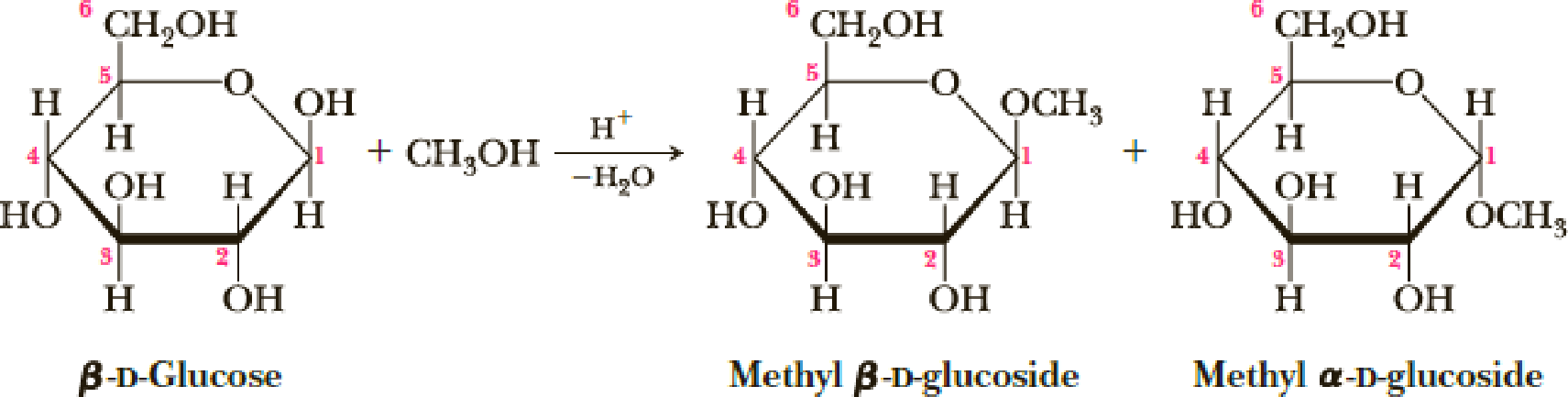
In these representations, the six-membered rings are drawn as planar hexagons.
- (a) Propose a mechanism for this conversion and account for the fact that only the ─OH on carbon 1 is transformed into an ─OCH3 group.
- (b) Draw the more stable chair conformation for each product.
- (c) Which of the two products has the chair conformation of greater stability? Explain.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Topic: Photochemistry and Photophysics of Supramolecules
Two cations that exchange an electron in an interface, the exchange density is worth 1.39 mA/cm2 and the current density is worth 15 mA/cm2 at 25°C. If the overvoltage is 0.14 V, calculate the reaction rate and symmetry factor. Data: R = 8,314 J mol-1 k-1: F = 96500 C
With the help of the Tafel line, it is estimated that the interchange density of the VO2+/VO2+ system on the carbon paper has a value of 3 mA cm-2. Calculate a) the current density if the voltage has a value of 1.6 mV and the temperature is 25°C. b) the beta value of the anódico process if the Tafel pendulum is 0.6 V at 25°C. Data: R = 8.314 JK-1mol-1, y F = 96485 C mol-1.
Chapter 16 Solutions
Organic Chemistry
Ch. 16.1 - Write the IUPAC name for each compound. Specify...Ch. 16.1 - Write structural formulas for all aldehydes with...Ch. 16.1 - Write the IUPAC name for each compound.Ch. 16.5 - Prob. 16.4PCh. 16.6 - Prob. 16.5PCh. 16.7 - Prob. 16.6PCh. 16.7 - Write a mechanism for the acid-catalyzed...Ch. 16.8 - Prob. 16.8PCh. 16.8 - The given mechanism of transamination reaction is...Ch. 16.8 - The given mechanism of transamination reaction is...
Ch. 16.8 - The given mechanism of transamination reaction is...Ch. 16.8 - Prob. DQCh. 16.8 - Prob. EQCh. 16.8 - The given mechanism of transamination reaction is...Ch. 16.9 - Predict the position of the following equilibrium.Ch. 16.9 - Draw a structural formula for the keto form of...Ch. 16.10 - Prob. 16.11PCh. 16.11 - What aldehyde or ketone gives these alcohols upon...Ch. 16.11 - Prob. 16.13PCh. 16 - Prob. 16.14PCh. 16 - Prob. 16.15PCh. 16 - The infrared spectrum of compound A, C6H12O, shows...Ch. 16 - Following are 1H-NMR spectra for compounds B...Ch. 16 - Draw structural formulas for the product formed by...Ch. 16 - Suggest a synthesis for the following alcohols...Ch. 16 - Show how to synthesize the following alcohol using...Ch. 16 - 1-Phenyl-2-butanol is used in perfumery. Show how...Ch. 16 - Prob. 16.22PCh. 16 - Draw structural formulas for (1) the...Ch. 16 - Show how to bring about the following conversions...Ch. 16 - Prob. 16.25PCh. 16 - Wittig reactions with the following -chloroethers...Ch. 16 - Prob. 16.27PCh. 16 - Prob. 16.28PCh. 16 - 5-Hydroxyhexanal forms a six-membered cyclic...Ch. 16 - Prob. 16.30PCh. 16 - Prob. 16.31PCh. 16 - Propose a mechanism to account for the formation...Ch. 16 - Prob. 16.33PCh. 16 - Prob. 16.34PCh. 16 - Show how to bring about the following conversion.Ch. 16 - A primary or secondary alcohol can be protected by...Ch. 16 - Prob. 16.37PCh. 16 - Prob. 16.38PCh. 16 - Prob. 16.39PCh. 16 - Prob. 16.40PCh. 16 - The following molecule belongs to a class of...Ch. 16 - When cis-2-decalone is dissolved in ether...Ch. 16 - Prob. 16.43PCh. 16 - Prob. 16.44PCh. 16 - The following bicyclic ketone has two -carbons and...Ch. 16 - Propose a mechanism for this reaction.Ch. 16 - The base-promoted rearrangement of an -haloketone...Ch. 16 - If the Favorskii rearrangement of...Ch. 16 - (R)-Pulegone, readily available from pennyroyal...Ch. 16 - (R)-Pulegone is converted to (R)-citronellic acid...Ch. 16 - Starting with cyclohexanone, show how to prepare...Ch. 16 - Show how to convert cyclopentanone to these...Ch. 16 - Prob. 16.53PCh. 16 - Prob. 16.54PCh. 16 - Prob. 16.55PCh. 16 - Following is the structural formula of Surfynol, a...Ch. 16 - Prob. 16.57PCh. 16 - Propose a mechanism for this isomerization.Ch. 16 - Starting with acetylene and 1-bromobutane as the...Ch. 16 - Prob. 16.60PCh. 16 - Prob. 16.61PCh. 16 - Prob. 16.62PCh. 16 - Prob. 16.63PCh. 16 - Prob. 16.64PCh. 16 - All rearrangements we have discussed so far have...Ch. 16 - In dilute aqueous base, (R)-glyceraldehyde is...Ch. 16 - Treatment of -D-glucose with methanol in the...Ch. 16 - Treating a Grignard reagent with carbon dioxide...Ch. 16 - Prob. 16.69PCh. 16 - Prob. 16.70PCh. 16 - Prob. 16.71PCh. 16 - Prob. 16.72PCh. 16 - Write the products of the following sequences of...Ch. 16 - Using your reaction roadmaps as a guide, show how...Ch. 16 - Using your reaction roadmaps as a guide, show how...Ch. 16 - Using your reaction roadmaps as a guide, show how...Ch. 16 - Using your reaction roadmaps as a guide, show how...Ch. 16 - Prob. 16.78PCh. 16 - Prob. 16.79PCh. 16 - Prob. 16.80PCh. 16 - Prob. 16.81P
Additional Science Textbook Solutions
Find more solutions based on key concepts
Describe the evolution of mammals, tracing their synapsid lineage from early amniote ancestors to true mammals....
Loose Leaf For Integrated Principles Of Zoology
How does the removal of hydrogen atoms from nutrient molecules result in a loss of energy from the nutrient mol...
SEELEY'S ANATOMY+PHYSIOLOGY
Choose the best answer to each of the following. Explain your reasoning. If Earth were twice as far as it actua...
Cosmic Perspective Fundamentals
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Hi can you please help me solve this problem? thank youarrow_forwardAn electrode process takes place at a metal-solution interface. Indicate the current condition that must be met for Faradaic rectification to occur.arrow_forwardAt a metal-solution interface, an electron is exchanged, and the symmetry factor beta < 0.5 is found in the Butler-Volmer equation. What does this indicate?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License