
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 16, Problem 16.70P
Interpretation Introduction
Interpretation:
The given reaction is
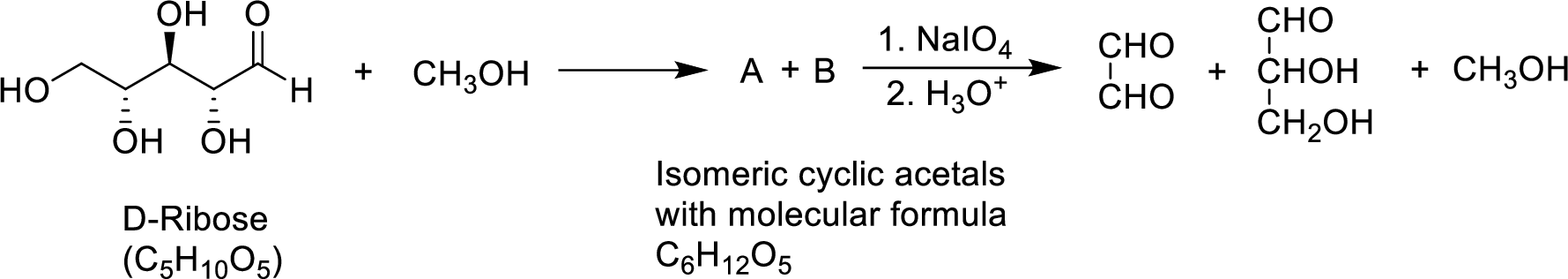
The cyclic hemiacetals formed from D-ribose are four-membered or five-membered or six-membered has to be given.
Concept Introduction:
Cyclic hemiacetals are formed by the intramolecular reaction of a carbonyl group with alcohol group.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
1. Determine the relationship between the following molecules as identical, diastereomers, or enantiomers (6
points, 2 points each).
OH
OH
OH
A-A
OH
HOT
HO-
ACHN
and
HO-
ACHN
OH
HO
HO
°
OH
and
OH
OH
SH
and
...SH
20,0
Complete the electron pushing mechanism to
y drawing the necomery unicaciones and carved on for
Step 1: Add curved arms for the tint step, traiment with NalilĻ. The Nation
458
Step 2: Added for the second step, inalment with), how the "counterion
bar
Step 3: Daw the products of the last simplom organic and one incoganic spacient, including all nonbonding
please provide the structure for this problem, thank you!
Chapter 16 Solutions
Organic Chemistry
Ch. 16.1 - Write the IUPAC name for each compound. Specify...Ch. 16.1 - Write structural formulas for all aldehydes with...Ch. 16.1 - Write the IUPAC name for each compound.Ch. 16.5 - Prob. 16.4PCh. 16.6 - Prob. 16.5PCh. 16.7 - Prob. 16.6PCh. 16.7 - Write a mechanism for the acid-catalyzed...Ch. 16.8 - Prob. 16.8PCh. 16.8 - The given mechanism of transamination reaction is...Ch. 16.8 - The given mechanism of transamination reaction is...
Ch. 16.8 - The given mechanism of transamination reaction is...Ch. 16.8 - Prob. DQCh. 16.8 - Prob. EQCh. 16.8 - The given mechanism of transamination reaction is...Ch. 16.9 - Predict the position of the following equilibrium.Ch. 16.9 - Draw a structural formula for the keto form of...Ch. 16.10 - Prob. 16.11PCh. 16.11 - What aldehyde or ketone gives these alcohols upon...Ch. 16.11 - Prob. 16.13PCh. 16 - Prob. 16.14PCh. 16 - Prob. 16.15PCh. 16 - The infrared spectrum of compound A, C6H12O, shows...Ch. 16 - Following are 1H-NMR spectra for compounds B...Ch. 16 - Draw structural formulas for the product formed by...Ch. 16 - Suggest a synthesis for the following alcohols...Ch. 16 - Show how to synthesize the following alcohol using...Ch. 16 - 1-Phenyl-2-butanol is used in perfumery. Show how...Ch. 16 - Prob. 16.22PCh. 16 - Draw structural formulas for (1) the...Ch. 16 - Show how to bring about the following conversions...Ch. 16 - Prob. 16.25PCh. 16 - Wittig reactions with the following -chloroethers...Ch. 16 - Prob. 16.27PCh. 16 - Prob. 16.28PCh. 16 - 5-Hydroxyhexanal forms a six-membered cyclic...Ch. 16 - Prob. 16.30PCh. 16 - Prob. 16.31PCh. 16 - Propose a mechanism to account for the formation...Ch. 16 - Prob. 16.33PCh. 16 - Prob. 16.34PCh. 16 - Show how to bring about the following conversion.Ch. 16 - A primary or secondary alcohol can be protected by...Ch. 16 - Prob. 16.37PCh. 16 - Prob. 16.38PCh. 16 - Prob. 16.39PCh. 16 - Prob. 16.40PCh. 16 - The following molecule belongs to a class of...Ch. 16 - When cis-2-decalone is dissolved in ether...Ch. 16 - Prob. 16.43PCh. 16 - Prob. 16.44PCh. 16 - The following bicyclic ketone has two -carbons and...Ch. 16 - Propose a mechanism for this reaction.Ch. 16 - The base-promoted rearrangement of an -haloketone...Ch. 16 - If the Favorskii rearrangement of...Ch. 16 - (R)-Pulegone, readily available from pennyroyal...Ch. 16 - (R)-Pulegone is converted to (R)-citronellic acid...Ch. 16 - Starting with cyclohexanone, show how to prepare...Ch. 16 - Show how to convert cyclopentanone to these...Ch. 16 - Prob. 16.53PCh. 16 - Prob. 16.54PCh. 16 - Prob. 16.55PCh. 16 - Following is the structural formula of Surfynol, a...Ch. 16 - Prob. 16.57PCh. 16 - Propose a mechanism for this isomerization.Ch. 16 - Starting with acetylene and 1-bromobutane as the...Ch. 16 - Prob. 16.60PCh. 16 - Prob. 16.61PCh. 16 - Prob. 16.62PCh. 16 - Prob. 16.63PCh. 16 - Prob. 16.64PCh. 16 - All rearrangements we have discussed so far have...Ch. 16 - In dilute aqueous base, (R)-glyceraldehyde is...Ch. 16 - Treatment of -D-glucose with methanol in the...Ch. 16 - Treating a Grignard reagent with carbon dioxide...Ch. 16 - Prob. 16.69PCh. 16 - Prob. 16.70PCh. 16 - Prob. 16.71PCh. 16 - Prob. 16.72PCh. 16 - Write the products of the following sequences of...Ch. 16 - Using your reaction roadmaps as a guide, show how...Ch. 16 - Using your reaction roadmaps as a guide, show how...Ch. 16 - Using your reaction roadmaps as a guide, show how...Ch. 16 - Using your reaction roadmaps as a guide, show how...Ch. 16 - Prob. 16.78PCh. 16 - Prob. 16.79PCh. 16 - Prob. 16.80PCh. 16 - Prob. 16.81P
Knowledge Booster
Similar questions
- Draw the Fischer projection from the skeletal structure shown below. HO OH OH OH OH H Q Drawing Atoms, Bonds and Rings Charges I ☐ T HO H H OH HO I CH2OH H OH Drag H OH -CH2OH CHO -COOH Undo Reset Remove Donearrow_forwardplease provide the structure for this problem, thank youarrow_forwardpresented by Morallen Lig Intermine the hand product for the given mution by adding atoms, bonds, nonhonding diarion panda скуль Step 3: Comp the draw the product Step 2: Agama workup Compithe 429 ملولةarrow_forward
- Reaction A 0,0arrow_forwardpresented by Morillon Leaning Predict the organic product for the min кусур HSC Adithane carved arnown to come than that to the condon slchroruis in acid in in aquishri with ноюarrow_forward6.15PM Sun Mar 30 K Draw the major product of this reaction. Include any relevant stereochemistry. Ignore inorganic byproducts. Problem 1 of O H [PhзPCH2CH3]*C|¯ NaH Drawing > Q Atoms, Bonds and Draw or tap a nearrow_forward
- 8:17 PM Sun Mar 30 Draw the major product of this reaction. Ignore inorganic byproducts. HSCH2CH2CH2SH, BF3 Probler Drawing Ato Bonds Clarrow_forwardpresented by Mr L How the coprion. (Il Done in no wraction, dew the starting redential) доarrow_forward8:16 PM Sun Mar 30 K Draw the major product of this reaction. Ignore inorganic byproducts. Proble 1. CH3MgBr 2. H3O+ F Drawingarrow_forward
- о но оarrow_forwardName the major organic product of the following action of 4-chloro-4-methyl-1-pentanol in neutral pollution 10+ Now the product. The product has a molecular formula f b. In a singly hain, the starting, material again converts into a secule with the molecular kormula CIO. but with comply Draw the major organic structure inhalationarrow_forwardMacmillan Learning Alcohols can be oxidized by chromic acid derivatives. One such reagent is pyridinium chlorochromate, (C,H,NH*)(CICTO3), commonly known as PCC. Draw the proposed (neutral) intermediate and the organic product in the oxidation of 1-butanol by PCC when carried out in an anhydrous solvent such as CH₂C₁₂. PCC Intermediate OH CH2Cl2 Draw the intermediate. Select Draw Templates More с H Cr о Product Draw the product. Erase Select Draw Templates More H о Erasearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning