
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 16, Problem 16.78P
(a)
Interpretation Introduction
Interpretation:
The product formed in the given reaction has to be drawn.
Concept Introduction:
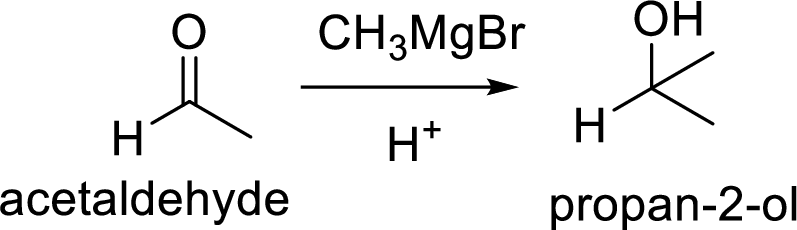
(b)
Interpretation Introduction
Interpretation:
The chiral center has to be labeled in cetirizine.
Concept Introduction:
Chiral center: A chiral center is defined as the tetrahedral carbon atom in an organic molecule that is connected to four non-identical groups/substituents. It is sometimes known as a stereo genic center.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Please help me answer these three questions. Required info should be in data table.
Draw the major organic substitution product or products for (2R,3S)-2-bromo-3-methylpentane reacting with the given
nucleophile. Clearly drawn the stereochemistry, including a wedged bond, a dashed bond and two in-plane bonds at each
stereogenic center. Omit any byproducts.
Bri
CH3CH2O-
(conc.)
Draw the major organic product or products.
Tartaric acid (C4H6O6) is a diprotic weak acid. A sample of 875 mg tartaric acid are dissolved in 100 mL water and titrated with 0.994 M NaOH.
How many mL of NaOH are needed to reach the first equivalence point?
How many mL of NaOH are needed to reach the second equivalence point?
Chapter 16 Solutions
Organic Chemistry
Ch. 16.1 - Write the IUPAC name for each compound. Specify...Ch. 16.1 - Write structural formulas for all aldehydes with...Ch. 16.1 - Write the IUPAC name for each compound.Ch. 16.5 - Prob. 16.4PCh. 16.6 - Prob. 16.5PCh. 16.7 - Prob. 16.6PCh. 16.7 - Write a mechanism for the acid-catalyzed...Ch. 16.8 - Prob. 16.8PCh. 16.8 - The given mechanism of transamination reaction is...Ch. 16.8 - The given mechanism of transamination reaction is...
Ch. 16.8 - The given mechanism of transamination reaction is...Ch. 16.8 - Prob. DQCh. 16.8 - Prob. EQCh. 16.8 - The given mechanism of transamination reaction is...Ch. 16.9 - Predict the position of the following equilibrium.Ch. 16.9 - Draw a structural formula for the keto form of...Ch. 16.10 - Prob. 16.11PCh. 16.11 - What aldehyde or ketone gives these alcohols upon...Ch. 16.11 - Prob. 16.13PCh. 16 - Prob. 16.14PCh. 16 - Prob. 16.15PCh. 16 - The infrared spectrum of compound A, C6H12O, shows...Ch. 16 - Following are 1H-NMR spectra for compounds B...Ch. 16 - Draw structural formulas for the product formed by...Ch. 16 - Suggest a synthesis for the following alcohols...Ch. 16 - Show how to synthesize the following alcohol using...Ch. 16 - 1-Phenyl-2-butanol is used in perfumery. Show how...Ch. 16 - Prob. 16.22PCh. 16 - Draw structural formulas for (1) the...Ch. 16 - Show how to bring about the following conversions...Ch. 16 - Prob. 16.25PCh. 16 - Wittig reactions with the following -chloroethers...Ch. 16 - Prob. 16.27PCh. 16 - Prob. 16.28PCh. 16 - 5-Hydroxyhexanal forms a six-membered cyclic...Ch. 16 - Prob. 16.30PCh. 16 - Prob. 16.31PCh. 16 - Propose a mechanism to account for the formation...Ch. 16 - Prob. 16.33PCh. 16 - Prob. 16.34PCh. 16 - Show how to bring about the following conversion.Ch. 16 - A primary or secondary alcohol can be protected by...Ch. 16 - Prob. 16.37PCh. 16 - Prob. 16.38PCh. 16 - Prob. 16.39PCh. 16 - Prob. 16.40PCh. 16 - The following molecule belongs to a class of...Ch. 16 - When cis-2-decalone is dissolved in ether...Ch. 16 - Prob. 16.43PCh. 16 - Prob. 16.44PCh. 16 - The following bicyclic ketone has two -carbons and...Ch. 16 - Propose a mechanism for this reaction.Ch. 16 - The base-promoted rearrangement of an -haloketone...Ch. 16 - If the Favorskii rearrangement of...Ch. 16 - (R)-Pulegone, readily available from pennyroyal...Ch. 16 - (R)-Pulegone is converted to (R)-citronellic acid...Ch. 16 - Starting with cyclohexanone, show how to prepare...Ch. 16 - Show how to convert cyclopentanone to these...Ch. 16 - Prob. 16.53PCh. 16 - Prob. 16.54PCh. 16 - Prob. 16.55PCh. 16 - Following is the structural formula of Surfynol, a...Ch. 16 - Prob. 16.57PCh. 16 - Propose a mechanism for this isomerization.Ch. 16 - Starting with acetylene and 1-bromobutane as the...Ch. 16 - Prob. 16.60PCh. 16 - Prob. 16.61PCh. 16 - Prob. 16.62PCh. 16 - Prob. 16.63PCh. 16 - Prob. 16.64PCh. 16 - All rearrangements we have discussed so far have...Ch. 16 - In dilute aqueous base, (R)-glyceraldehyde is...Ch. 16 - Treatment of -D-glucose with methanol in the...Ch. 16 - Treating a Grignard reagent with carbon dioxide...Ch. 16 - Prob. 16.69PCh. 16 - Prob. 16.70PCh. 16 - Prob. 16.71PCh. 16 - Prob. 16.72PCh. 16 - Write the products of the following sequences of...Ch. 16 - Using your reaction roadmaps as a guide, show how...Ch. 16 - Using your reaction roadmaps as a guide, show how...Ch. 16 - Using your reaction roadmaps as a guide, show how...Ch. 16 - Using your reaction roadmaps as a guide, show how...Ch. 16 - Prob. 16.78PCh. 16 - Prob. 16.79PCh. 16 - Prob. 16.80PCh. 16 - Prob. 16.81P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Including activity, calculate the solubility of Pb(IO3)2 in a matrix of 0.020 M Mg(NO3)2.arrow_forwardIncluding activity coefficients, find [Hg22+] in saturated Hg2Br2 in 0.00100 M KBr.arrow_forwardIncluding activity, calculate the pH of a 0.010 M HCl solution with an ionic strength of 0.10 M.arrow_forward
- Can I please get the graph 1: Concentration vs. Density?arrow_forwardOrder the following series of compounds from highest to lowest reactivity to electrophilic aromatic substitution, explaining your answer: 2-nitrophenol, p-Toluidine, N-(4-methylphenyl)acetamide, 4-methylbenzonitrile, 4-(trifluoromethyl)benzonitrile.arrow_forwardOrdene la siguiente serie de compuestos de mayor a menor reactividad a la sustitución aromática electrofílica, explicando su respuesta: ácido bencenosulfónico, fluorobenceno, etilbenceno, clorobenceno, terc-butilbenceno, acetofenona.arrow_forward
- Can I please get all final concentrations please!arrow_forwardState the detailed mechanism of the reaction of benzene with isopropanol in sulfuric acid.arrow_forwardDo not apply the calculations, based on the approximation of the stationary state, to make them perform correctly. Basta discard the 3 responses that you encounter that are obviously erroneous if you apply the formula to determine the speed of a reaction. For the decomposition reaction of N2O5(g): 2 N2O5(g) · 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 -> NO2 + NO3_(K1) NO2 + NO3 →> N2O5 (k-1) → NO2 + NO3 → NO2 + O2 + NO (K2) NO + N2O5 → NO2 + NO2 + NO2 (K3) Give the expression for the acceptable rate. (A). d[N₂O] dt = -1 2k,k₂[N205] k₁+k₂ d[N₂O5] (B). dt =-k₁[N₂O₂] + k₁[NO2][NO3] - k₂[NO2]³ (C). d[N₂O] dt =-k₁[N₂O] + k₁[N205] - K3 [NO] [N205] (D). d[N2O5] =-k₁[NO] - K3[NO] [N₂05] dtarrow_forward
- A 0.10 M solution of acetic acid (CH3COOH, Ka = 1.8 x 10^-5) is titrated with a 0.0250 M solution of magnesium hydroxide (Mg(OH)2). If 10.0 mL of the acid solution is titrated with 20.0 mL of the base solution, what is the pH of the resulting solution?arrow_forwardFor the decomposition reaction of N2O5(g): 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 NO2 + NO3 (K1) | NO2 + NO3 → N2O5 (k-1) | NO2 + NO3 NO2 + O2 + NO (k2) | NO + N2O51 NO2 + NO2 + NO2 (K3) → Give the expression for the acceptable rate. → → (A). d[N205] dt == 2k,k₂[N₂O₂] k₁+k₁₂ (B). d[N2O5] =-k₁[N₂O] + k₁[NO₂] [NO3] - k₂[NO₂]³ dt (C). d[N2O5] =-k₁[N₂O] + k [NO] - k₂[NO] [NO] d[N2O5] (D). = dt = -k₁[N2O5] - k¸[NO][N₂05] dt Do not apply the calculations, based on the approximation of the stationary state, to make them perform correctly. Basta discard the 3 responses that you encounter that are obviously erroneous if you apply the formula to determine the speed of a reaction.arrow_forwardFor the decomposition reaction of N2O5(g): 2 N2O5(g) → 4 NO2(g) + O2(g), the following mechanism has been proposed: N2O5 NO2 + NO3 (K1) | NO2 + NO3 → N2O5 (k-1) | NO2 + NO3 NO2 + O2 + NO (k2) | NO + N2O51 NO2 + NO2 + NO2 (K3) → Give the expression for the acceptable rate. → → (A). d[N205] dt == 2k,k₂[N₂O₂] k₁+k₁₂ (B). d[N2O5] =-k₁[N₂O] + k₁[NO₂] [NO3] - k₂[NO₂]³ dt (C). d[N2O5] =-k₁[N₂O] + k [NO] - k₂[NO] [NO] d[N2O5] (D). = dt = -k₁[N2O5] - k¸[NO][N₂05] dt Do not apply the calculations, based on the approximation of the stationary state, to make them perform correctly. Basta discard the 3 responses that you encounter that are obviously erroneous if you apply the formula to determine the speed of a reaction.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning