
EBK ORGANIC AND BIOLOGICAL CHEMISTRY
7th Edition
ISBN: 9780100547742
Author: STOKER
Publisher: YUZU
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 15.8, Problem 3QQ
Interpretation Introduction
Interpretation: To determine the reactant in the first step of the sulfate assimilation process.
Concept introduction: Sulfate assimilation is the process used for the production of hydrogen sulfide. The starting material for sulfate assimilation is sulfate ion. It is an oxidation-reduction process as the sulfur in sulfate ion is in oxidized form and in hydrogen sulfide it is present in the reduced form.
The conversion of sulfate ion to sulfide ion via sulfate assimilation occurs in 4 steps. These steps are as follows:
Step 1:
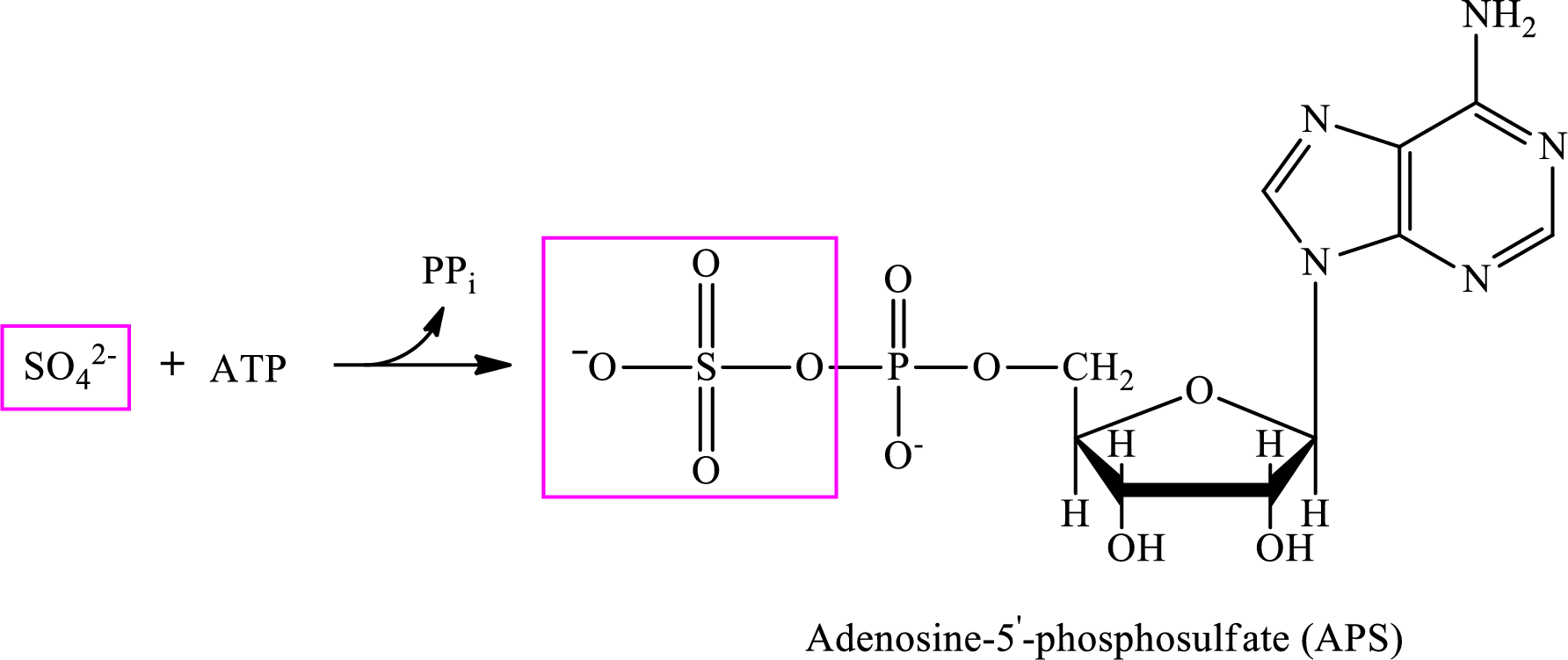
Step 2:
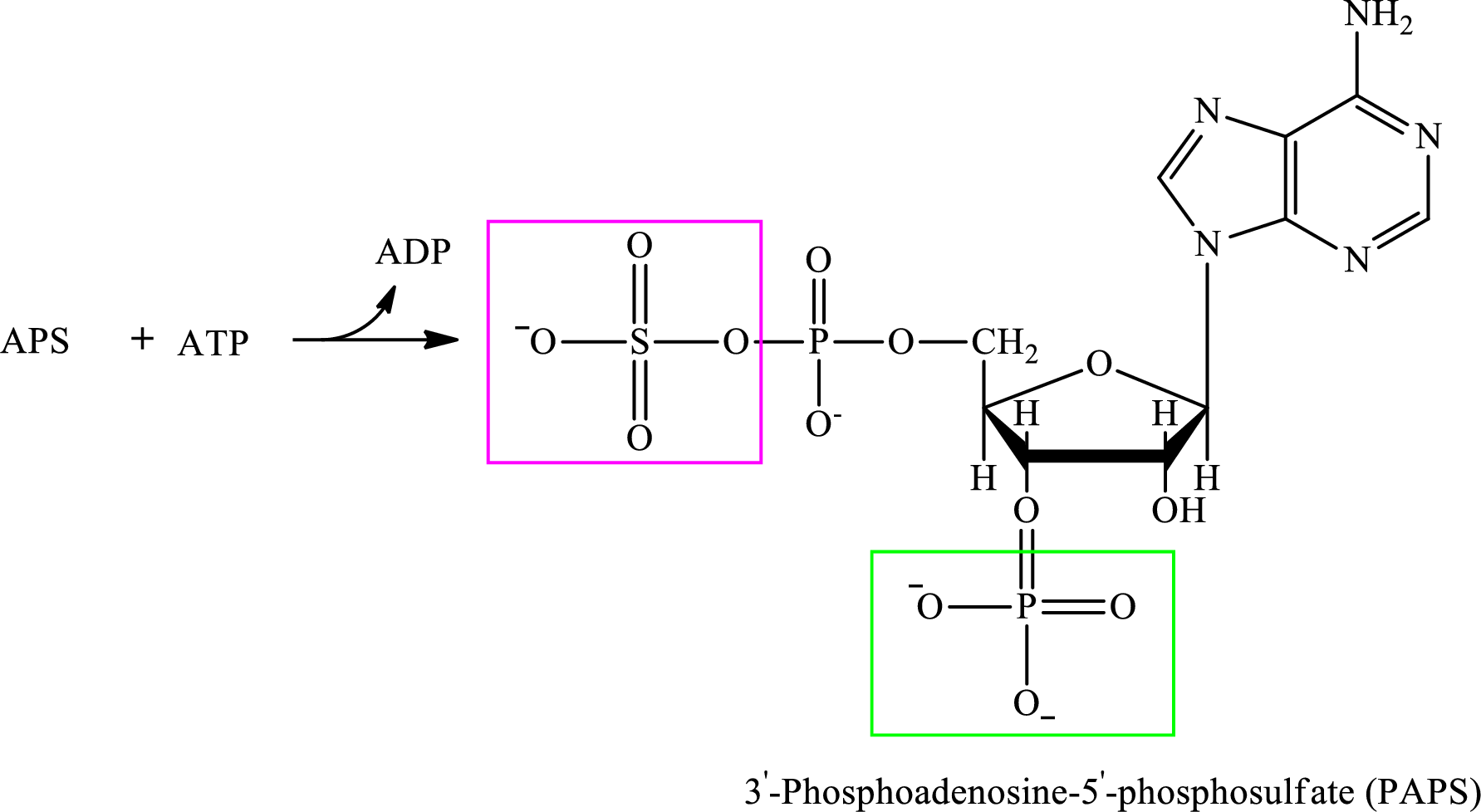
Step 3:
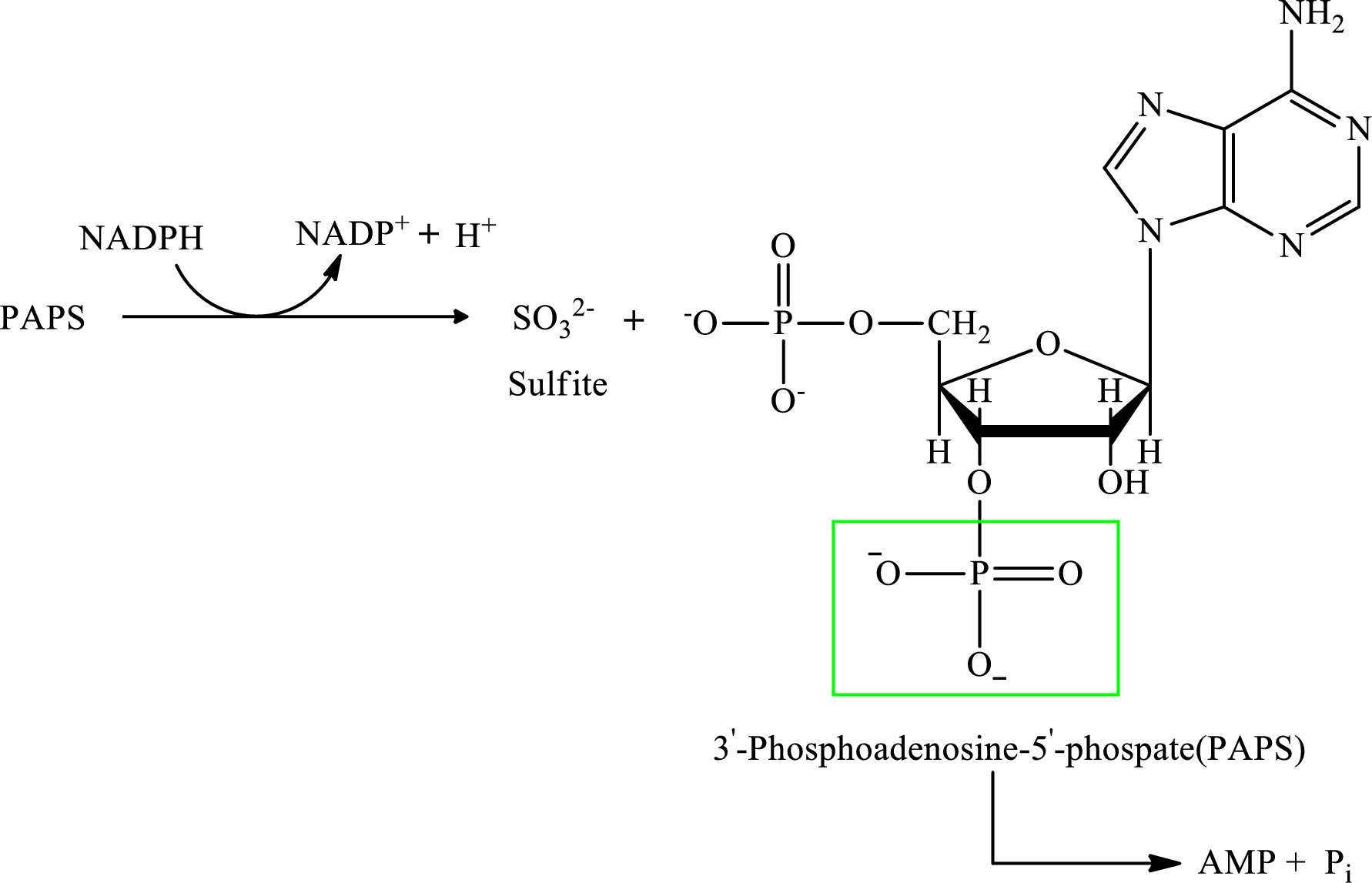
Step 4:

Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Draw the Fischer projection of D-fructose.
Click and drag to start drawing a
structure.
Skip Part
Check
AP
14
tv
SC
F1
F2
80
F3
a
F4
!
2
#
3
CF
F5
75
Ax
MacBook Air
894
$
5olo
%
Λ
6 >
W
F6
K
F7
&
Consider this step in a radical reaction:
Y
What type of step is this? Check all that apply.
Draw the products of the step on the right-hand side of the drawing area
below. If more than one set of products is possible, draw any set.
Also, draw the mechanism arrows on the left-hand side of the drawing
area to show how this happens.
ionization
propagation
initialization
passivation
none of the above
22.16 The following groups are ortho-para directors.
(a)
-C=CH₂
H
(d)
-Br
(b)
-NH2
(c)
-OCHS
Draw a contributing structure for the resonance-stabilized cation formed during elec-
trophilic aromatic substitution that shows the role of each group in stabilizing the
intermediate by further delocalizing its positive charge.
22.17 Predict the major product or products from treatment of each compound with
Cl₁/FeCl₂-
OH
(b)
NO2
CHO
22.18 How do you account for the fact that phenyl acetate is less reactive toward electro-
philic aromatic substitution than anisole?
Phenyl acetate
Anisole
CH
(d)
Chapter 15 Solutions
EBK ORGANIC AND BIOLOGICAL CHEMISTRY
Ch. 15.1 - Which of the following statements about dietary...Ch. 15.1 - Dietary protein materials as they leave the...Ch. 15.1 - Prob. 3QQCh. 15.1 - Which of the following is not a proteolytic...Ch. 15.2 - The dominant use for the amino acids of the amino...Ch. 15.2 - The most abundant amino acid in the amino acid...Ch. 15.2 - Prob. 3QQCh. 15.3 - The reactants in a transamination reaction are a....Ch. 15.3 - Prob. 2QQCh. 15.3 - Prob. 3QQ
Ch. 15.3 - Prob. 4QQCh. 15.3 - Prob. 5QQCh. 15.3 - Prob. 6QQCh. 15.4 - Prob. 1QQCh. 15.4 - Prob. 2QQCh. 15.4 - Prob. 3QQCh. 15.4 - Prob. 4QQCh. 15.4 - Prob. 5QQCh. 15.4 - In the urea cycle, the urea-producing step...Ch. 15.5 - Which of the following statements concerning the...Ch. 15.5 - Prob. 2QQCh. 15.5 - Which of the following statements concerning the...Ch. 15.5 - Prob. 4QQCh. 15.6 - Prob. 1QQCh. 15.6 - Prob. 2QQCh. 15.6 - Prob. 3QQCh. 15.7 - Prob. 1QQCh. 15.7 - Prob. 2QQCh. 15.7 - In the degradation of heme, which of the following...Ch. 15.7 - In the degradation of heme, the iron atom present...Ch. 15.8 - In degradation of the sulfur-containing amino acid...Ch. 15.8 - Prob. 2QQCh. 15.8 - Prob. 3QQCh. 15.8 - Prob. 4QQCh. 15.9 - Prob. 1QQCh. 15.9 - Prob. 2QQCh. 15.9 - Prob. 3QQCh. 15.10 - Transamination reactions require the cofactor PLP...Ch. 15.10 - Prob. 2QQCh. 15.10 - Prob. 3QQCh. 15 - Prob. 15.1EPCh. 15 - Indicate whether each of the following aspects of...Ch. 15 - Prob. 15.3EPCh. 15 - Prob. 15.4EPCh. 15 - Prob. 15.5EPCh. 15 - Prob. 15.6EPCh. 15 - Prob. 15.7EPCh. 15 - Prob. 15.8EPCh. 15 - Prob. 15.9EPCh. 15 - Prob. 15.10EPCh. 15 - Prob. 15.11EPCh. 15 - Prob. 15.12EPCh. 15 - Prob. 15.13EPCh. 15 - Indicate whether each of the following statements...Ch. 15 - Prob. 15.15EPCh. 15 - Prob. 15.16EPCh. 15 - Prob. 15.17EPCh. 15 - What are the four major uses for amino acids...Ch. 15 - With the help of Table 26-1, classify each of the...Ch. 15 - Prob. 15.20EPCh. 15 - Prob. 15.21EPCh. 15 - Prob. 15.22EPCh. 15 - Prob. 15.23EPCh. 15 - Prob. 15.24EPCh. 15 - Prob. 15.25EPCh. 15 - Prob. 15.26EPCh. 15 - Prob. 15.27EPCh. 15 - Prob. 15.28EPCh. 15 - Prob. 15.29EPCh. 15 - Prob. 15.30EPCh. 15 - Prob. 15.31EPCh. 15 - Prob. 15.32EPCh. 15 - Prob. 15.33EPCh. 15 - Prob. 15.34EPCh. 15 - Prob. 15.35EPCh. 15 - Prob. 15.36EPCh. 15 - Prob. 15.37EPCh. 15 - Prob. 15.38EPCh. 15 - Prob. 15.39EPCh. 15 - Prob. 15.40EPCh. 15 - Prob. 15.41EPCh. 15 - Prob. 15.42EPCh. 15 - Draw the structure of the -keto acid produced from...Ch. 15 - Draw the structure of the -keto acid produced from...Ch. 15 - Prob. 15.45EPCh. 15 - Prob. 15.46EPCh. 15 - Prob. 15.47EPCh. 15 - Prob. 15.48EPCh. 15 - Prob. 15.49EPCh. 15 - Prob. 15.50EPCh. 15 - Prob. 15.51EPCh. 15 - Prob. 15.52EPCh. 15 - Prob. 15.53EPCh. 15 - Prob. 15.54EPCh. 15 - What is a carbamoyl group?Ch. 15 - Prob. 15.56EPCh. 15 - Prob. 15.57EPCh. 15 - Prob. 15.58EPCh. 15 - Prob. 15.59EPCh. 15 - Prob. 15.60EPCh. 15 - Prob. 15.61EPCh. 15 - Prob. 15.62EPCh. 15 - Prob. 15.63EPCh. 15 - Prob. 15.64EPCh. 15 - Prob. 15.65EPCh. 15 - Prob. 15.66EPCh. 15 - Prob. 15.67EPCh. 15 - Prob. 15.68EPCh. 15 - Prob. 15.69EPCh. 15 - Prob. 15.70EPCh. 15 - Prob. 15.71EPCh. 15 - Prob. 15.72EPCh. 15 - Prob. 15.73EPCh. 15 - Prob. 15.74EPCh. 15 - Prob. 15.75EPCh. 15 - Prob. 15.76EPCh. 15 - Prob. 15.77EPCh. 15 - Prob. 15.78EPCh. 15 - Prob. 15.79EPCh. 15 - Prob. 15.80EPCh. 15 - Prob. 15.81EPCh. 15 - Prob. 15.82EPCh. 15 - Prob. 15.83EPCh. 15 - Prob. 15.84EPCh. 15 - Prob. 15.85EPCh. 15 - Prob. 15.86EPCh. 15 - Prob. 15.87EPCh. 15 - What is the starting material for the biosynthesis...Ch. 15 - Prob. 15.89EPCh. 15 - Prob. 15.90EPCh. 15 - Prob. 15.91EPCh. 15 - Prob. 15.92EPCh. 15 - Prob. 15.93EPCh. 15 - What are the structural differences between...Ch. 15 - Prob. 15.95EPCh. 15 - Prob. 15.96EPCh. 15 - Which bile pigment is responsible for the yellow...Ch. 15 - Prob. 15.98EPCh. 15 - Prob. 15.99EPCh. 15 - Prob. 15.100EPCh. 15 - Prob. 15.101EPCh. 15 - Prob. 15.102EPCh. 15 - Prob. 15.103EPCh. 15 - Prob. 15.104EPCh. 15 - Prob. 15.105EPCh. 15 - Indicate whether each of the following statements...Ch. 15 - Prob. 15.107EPCh. 15 - Prob. 15.108EPCh. 15 - Prob. 15.109EPCh. 15 - Prob. 15.110EPCh. 15 - Prob. 15.111EPCh. 15 - Prob. 15.112EPCh. 15 - Prob. 15.113EPCh. 15 - Prob. 15.114EPCh. 15 - Prob. 15.115EPCh. 15 - Prob. 15.116EP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Show how to convert ethyl benzene to (a) 2,5-dichlorobenzoic acid and (b) 2,4-dichlorobenzoic acid.arrow_forwardHelp me solve this problem. Thank you in advance.arrow_forward22.7 Predict the monoalkylated products of the following reactions with benzene. (a) AlCl3 Ya (b) AlCl3 (c) H3PO4 (d) 22.8 Think-Pair-Share AICI3 The reaction below is a common electrophilic aromatic substitution. SO3 H₂SO4 SO₂H (a) Draw the reaction mechanism for this reaction using HSO,+ as the electrophile. (b) Sketch the reaction coordinate diagram, where the product is lower in energy than the starting reactant. (c) Which step in the reaction mechanism is highest in energy? Explain. (d) Which of the following reaction conditions could be used in an electrophilic aro- matic substitution with benzene to provide substituted phenyl derivatives? (i) AICI3 HNO3 H₂SO4 K2Cr2O7 (iii) H₂SO4 (iv) H₂PO₁arrow_forward
- Is an acid-base reaction the only type of reaction that would cause leavening products to rise?arrow_forwardHelp me understand this! Thank you in advance.arrow_forward22.22 For each compound, indicate which group on the ring is more strongly activating and then draw a structural formula of the major product formed by nitration of the compound. Br CHO (a) CH3 (b) (c) CHO CH3 SO₂H (d) ☑ OCHS NO₂ (e) (f) CO₂H NHCOCH3 NHCOCH, (h) CHS 22.23 The following molecules each contain two aromatic rings. (b) 000-100- H3C (a) (c) Which ring in each undergoes electrophilic aromatic substitution more readily? Draw the major product formed on nitration.arrow_forward
- V Consider this step in a radical reaction: Br: ? What type of step is this? Check all that apply. Draw the products of the step on the right-hand side of the drawing area below. If more than one set of products is possible, draw any set. Also, draw the mechanism arrows on the left-hand side of the drawing area to show how this happens. ⚫ionization termination initialization neutralization none of the abc Explanation Check 80 Ο F3 F1 F2 2 F4 01 % do5 $ 94 #3 X 5 C MacBook Air 25 F5 F6 66 ©2025 ˇ F7 29 & 7 8arrow_forwardShow how to convert ethyl benzene to (a) 2,5-dichlorobenzoic acid and (b) 2,4-dichlorobenzoic acid.arrow_forwardno aiarrow_forward
- Polymers may be composed of thousands of monomers. Draw three repeat units (trimer) of the polymer formed in this reaction. Assume there are hydrogen atoms there are hydrogen atoms on the two ends of the trimer. Ignore inorganic byproducts.arrow_forwardDraw a tetramer if this alternating copolymer pleasearrow_forwardDraw the monomers required to synthesize this condensation polymer.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning