
Concept explainers
(a)
Interpretation: To characterize oxaloacetate as a possible reactant, product, or enzyme involved in transamination, oxidative deamination, or both transamination and oxidative deamination.
Concept introduction: Transamination reaction is a biochemical reaction that involves the transfer of an amino group. In transamination reaction exchange of an amino group from an
A biochemical reaction in which an
A general oxidative deamination reaction is as follows:
(a)
Answer to Problem 15.46EP
Oxaloacetate can function as a product both in transamination reaction oxidative deamination reactions.
Explanation of Solution
Oxaloacetate is a keto acid. In both transamination and oxidative deamination reaction exchange of an amino group from an
Oxaloacetate is a corresponding keto acid of aspartate. Both of them have the same carbon skeleton. Aspartate gives oxaloacetate product in both transamination and oxidative deamination reaction.
(b)
Interpretation: To characterize aspartate as a possible reactant, product, or enzyme involved in transamination, oxidative deamination, or both transamination and oxidative deamination.
Concept introduction: Transamination reaction is a biochemical reaction that involves the transfer of an amino group. In transamination reaction exchange of an amino group from an
A biochemical reaction in which an
A general oxidative deamination reaction is as follows:
(b)
Answer to Problem 15.46EP
Aspartate acts as a reactant in transamination reaction.
Explanation of Solution
In transamination reaction exchange of an amino group from an
Aspartate is an
(c)
Interpretation: To characterize glutamate aminotransferase as a possible reactant, product, or enzyme involved in transamination, oxidative deamination, or both transamination and oxidative deamination.
Concept introduction: Transamination reaction is a biochemical reaction that involves the transfer of an amino group. In transamination reaction exchange of an amino group from an
A biochemical reaction in which an
A general oxidative deamination reaction is as follows:
(c)
Answer to Problem 15.46EP
Glutamate aminotransferase is the enzyme involved in the transamination reaction of glutamate to give
Explanation of Solution
Aminotransferases are enzymes used for transamination reaction. These catalyze the interchange of an amino group from an
(d)
Interpretation: To characterize
Concept introduction: Transamination reaction is a biochemical reaction that involves the transfer of an amino group. In transamination reaction exchange of an amino group from an
A biochemical reaction in which an
A general oxidative deamination reaction is as follows:
(d)
Answer to Problem 15.46EP
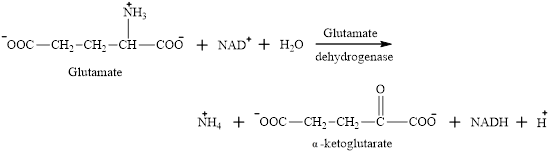
Water is one of the reactants in oxidative deamination reaction of glutamate.
Explanation of Solution
Glutamate is an
Want to see more full solutions like this?
Chapter 15 Solutions
EBK ORGANIC AND BIOLOGICAL CHEMISTRY
- For CARS, which statement is not true regarding its advantages? a) Contrast signal based on vibrational characteristics, no need for fluorescent tagging. b) Stronger signals than spontaneous Raman. c) Suffers from fluorescence interference, because CARS signal is at high frequency. d) Faster, more efficient imaging for real-time analysis. e) Higher resolution than spontaneous Raman microscopy.arrow_forwardDraw the major product of the Claisen condensation reaction between two molecules of this ester. Ignore inorganic byproducts. Incorrect, 5 attempts remaining 1. NaOCH3/CH3OH 2. Acidic workup Select to Draw O Incorrect, 5 attempts remaining The total number of carbons in the parent chain is incorrect. Review the reaction conditions including starting materials and/or intermediate structures and recount the number of carbon atoms in the parent chain of your structure. OKarrow_forwardUsing a cell of known pathlength b = 1.25115 x 10-3 cm, a water absorption spectrum was measured. The band at 1645 cm-1, assigned to the O-H bending, showed an absorbance, A, of 1.40. a) Assuming that water density is 1.00 g/mL, calculate the water molar concentration c (hint: M= mole/L) b) Calculate the molar absorptivity, a, of the 1645 cm-1 band c) The transmitted light, I, can be written as I= Ioexp(-xb), where x is the absorption coefficient (sometimes designated as alpha), Io is the input light, and b is the cell pathlength. Prove that x= (ln10)*x*c d) Calculate x for the 1645 cm-1 bandarrow_forward
- Convert 1.38 eV into wavelength (nm) and wavenumber (cm-1) (c = 2.998 x 108 m/s; h = 6.626 x 10-34 J*s).arrow_forwardCan you help me understand the CBC method on metal bridging by looking at this problem?arrow_forwardA partir de Aluminio y Co(NO3)2ꞏ6H2O, indicar las reacciones a realizar para obtener Azul de Thenard (Al2CoO4).arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





