
Interpretation:
To state the drawbacks of the reaction of
Concept introduction:
The synthesis of primary amine can be done by using three different methods.
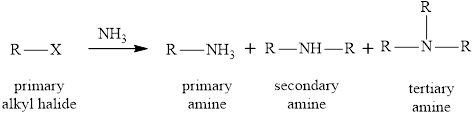
(1) In first method reaction of primary alkyl halide with ammonia is used as to prepare primary alkyl halide. This reaction is a nucleophilic substitution reaction in which ammonia molecule acts as a nucleophile and alkyl halide as the substrate. The products obtained after the reaction are mixture of primary, secondary and tertiary amines. Therefore, this method is not useful for the preparation of primary amines. The reaction equation is written as,
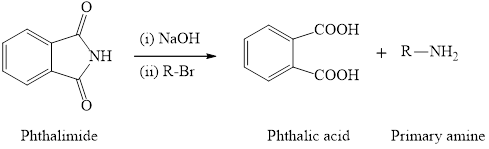
(2) The synthesis of primary amine is done by using phthalimide and primary alkyl halide in presence of hydroxide base. The important point for this reaction is the phthalimide group has only one hydrogen atom which is attached to nitrogen and can be replaced by alkyl group. Therefore, only one alkyl group can be substitued to the nitrogen atom and so only primary amine will form as the product. The general reaction equation is written as,
(3) The reaction of primary alkyl halide with azide ion forms alkyl azide which after catalytic hydrogenation reaction gives primary amine. This is the best method for the preparation of primary amines as the side product obtained is nitrogen gas which can be seperated from the primary amine easily. The reaction equation is written as,
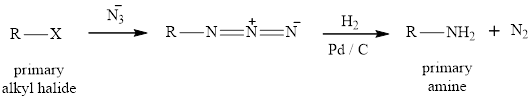
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
EBK ORGANIC CHEMISTRY
- identify the carbonyl compound that is incapable of forming an enolate ionarrow_forwardpredict the product formed by the reaction of one mole each of cyclohex-2-en-1-one and lithium diethylcuprate. Assume a hydrolysis step follows the additionarrow_forwardPlease handwriting for questions 1 and 3arrow_forward
- Is (CH3)3NHBr an acidic or basic salt? What happens when dissolved in aqueous solution? Doesn't it lose a Br-? Does it interact with the water? Please advise.arrow_forward© Macmilla Finish resonance structure 3 Select Draw Templates More C H N 0 H H S Erase Which structure is the most stable (lowest energy) resonance contributor? The structure with the positive charge on nitrogen and negative charges on oxygen and sulfur. All structures are equal in stability. The structure with the positive charge on nitrogen and negative charges on sulfur and carbon. The structure with the positive charge on nitrogen and negative charges on oxygen and carbon. Q2Qarrow_forwardThree pure compounds are formed when 1.00 g samples of element x combine with, respectively, 0.472 g, 0.630 g, and 0.789 g of element z. The first compound has the formula x2Z3. find the empricial formula of the other two compoundsarrow_forward
- Draw the product and the mechanism A. excess H*; 人 OH H*; B. C. D. excess OH ✓ OH H*; H₂O 1. LDA 2. H*arrow_forwardIn reactions whose kinetic equation is v = k[A]m, the rate coefficient k is always positive. Is this correct?arrow_forwardIf the concentration of A decreases exponentially with time, what is the rate equation? (A). -d[A] (B). dt d[A] = k[A] e-kt dtarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning



