
EBK ORGANIC CHEMISTRY
8th Edition
ISBN: 8220102744127
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15, Problem 60P
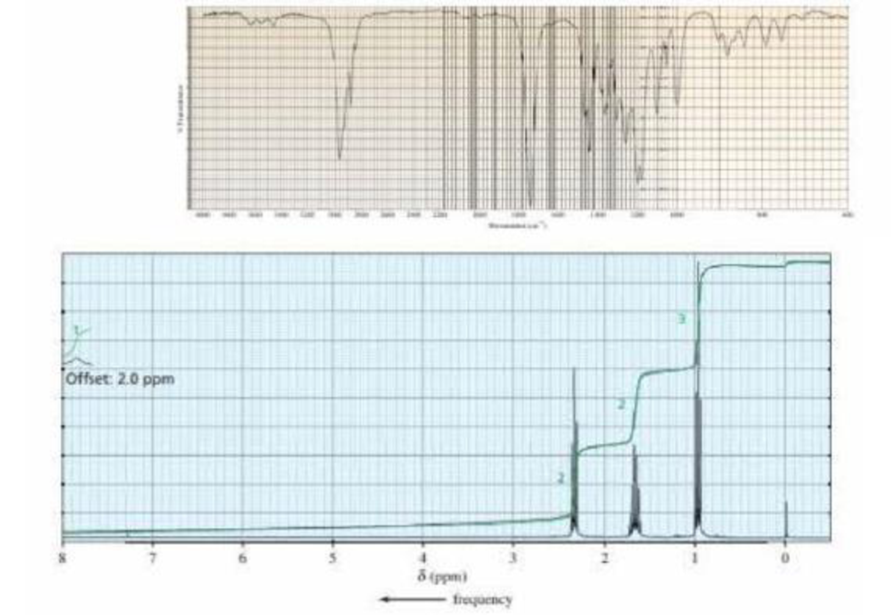
A compound with molecular formula C5H10O2 gives the following IR spectrum. When it undergoes acid-catalyzed hydrolysis, the compound with the 1H NMR spectrum shown below is formed. Identify the compounds.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Don't used hand raiting and don't used Ai solution
I don't understand what to put for final step. Does that just mean termination? And would a radical form when I add bromine to ch2 between the rings?
None
Chapter 15 Solutions
EBK ORGANIC CHEMISTRY
Ch. 15.1 - The aromas of many flowers and fruits are due to...Ch. 15.1 - Name the following:Ch. 15.1 - Prob. 3PCh. 15.2 - Which is longer, the carbon-oxygen single bond in...Ch. 15.2 - There are three carbon-oxygen bonds in methyl...Ch. 15.2 - Prob. 6PCh. 15.4 - a. What is the product of the reaction of acetyl...Ch. 15.4 - What is the product of an acyl substitution...Ch. 15.5 - a. Which compound has the stretching vibration for...Ch. 15.5 - Using the pKa values listed in Table 15.1, predict...
Ch. 15.5 - Is the following statement true or false? If the...Ch. 15.6 - Starting with acetyl chloride, what neutral...Ch. 15.6 - Prob. 13PCh. 15.7 - Starting with methyl acetate, what neutral...Ch. 15.7 - We saw that it is necessary to use excess amine in...Ch. 15.7 - Prob. 17PCh. 15.7 - Which ester hydrolyzes more rapidly? a. methyl...Ch. 15.7 - a. state three factors that cause the uncatalyzed...Ch. 15.8 - Prob. 21PCh. 15.8 - Using the mechanism for the acid-catalyzed...Ch. 15.8 - Prob. 23PCh. 15.8 - Show the mechanism for the acid-catalyzed...Ch. 15.8 - Prob. 25PCh. 15.8 - Write the mechanism for the acid-catalyzed...Ch. 15.8 - Write the mechanism for the acid-catalyzed...Ch. 15.9 - Prob. 28PCh. 15.9 - Prob. 29PCh. 15.10 - Show how each of the following esters could he...Ch. 15.10 - Prob. 32PCh. 15.11 - Prob. 33PCh. 15.11 - Which of the following reactions leads to the...Ch. 15.12 - Prob. 35PCh. 15.12 - Prob. 36PCh. 15.13 - Prob. 37PCh. 15.14 - Prob. 38PCh. 15.14 - Prob. 39PCh. 15.15 - Prob. 40PCh. 15.15 - Which alkyl halides from the carboxylic acids...Ch. 15.16 - Prob. 43PCh. 15.16 - Prob. 44PCh. 15.16 - Prob. 45PCh. 15.17 - Prob. 46PCh. 15.18 - How could you synthesize the following compounds...Ch. 15 - Prob. 48PCh. 15 - Name the following:Ch. 15 - Prob. 50PCh. 15 - What compound are obtained from the fallowing...Ch. 15 - a. Rank the following esters in order of...Ch. 15 - Because bromocyclohexane is a secondary alkyl...Ch. 15 - a. Which compound would you expect to have a...Ch. 15 - How could you use 1H NMR spectroscopy to...Ch. 15 - Rank the following compounds in order of...Ch. 15 - Prob. 57PCh. 15 - Prob. 58PCh. 15 - Prob. 59PCh. 15 - A compound with molecular formula C5H10O2 gives...Ch. 15 - Prob. 61PCh. 15 - Prob. 62PCh. 15 - Prob. 63PCh. 15 - Prob. 64PCh. 15 - Prob. 65PCh. 15 - Prob. 66PCh. 15 - Two products, A and B, are obtained from the...Ch. 15 - Prob. 68PCh. 15 - Prob. 69PCh. 15 - Prob. 70PCh. 15 - Prob. 71PCh. 15 - Prob. 72PCh. 15 - When treated with an equivalent of methanol,...Ch. 15 - a. Identify the two products obtained from the...Ch. 15 - Prob. 75PCh. 15 - Prob. 76PCh. 15 - a. When a carboxylic acid is dissolved in...Ch. 15 - Prob. 78PCh. 15 - Identity the major and minor products of the...Ch. 15 - When a compound with molecular formula C11H14O2...Ch. 15 - Prob. 81PCh. 15 - Prob. 82PCh. 15 - Prob. 83PCh. 15 - The 1H NMR spectra for two esters with molecular...Ch. 15 - Show how the following compounds could be prepared...Ch. 15 - Prob. 86PCh. 15 - Prob. 87PCh. 15 - The intermediate shown here is formed during the...Ch. 15 - Prob. 89PCh. 15 - Propose a mechanism that accounts for the...Ch. 15 - Catalytic antibodies catalyze a reaction by...Ch. 15 - Prob. 92P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- X Draw the major products of the elimination reaction below. If elimination would not occur at a significant rate, check the box under the drawing area instead. ది www. Cl + OH Elimination will not occur at a significant rate. Click and drag to start drawing a structure.arrow_forwardNonearrow_forward1A H 2A Li Be Use the References to access important values if needed for this question. 8A 3A 4A 5A 6A 7A He B C N O F Ne Na Mg 3B 4B 5B 6B 7B 8B-1B 2B Al Si P 1B 2B Al Si P S Cl Ar K Ca Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se Br Kr Rb Sr Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te I Xe * Cs Ba La Hf Ta W Re Os Ir Pt Au Hg Tl Pb Bi Po At Rn Fr Ra Ac Rf Ha ****** Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr Analyze the following reaction by looking at the electron configurations given below each box. Put a number and a symbol in each box to show the number and kind of the corresponding atom or ion. Use the smallest integers possible. cation anion + + Shell 1: 2 Shell 2: 8 Shell 3: 1 Shell 1 : 2 Shell 2 : 6 Shell 1 : 2 Shell 2: 8 Shell 1: 2 Shell 2: 8arrow_forward
- Nonearrow_forwardIV. Show the detailed synthesis strategy for the following compounds. a. CH3CH2CH2CH2Br CH3CH2CCH2CH2CH3arrow_forwardDo the electrons on the OH participate in resonance with the ring through a p orbital? How many pi electrons are in the ring, 4 (from the two double bonds) or 6 (including the electrons on the O)?arrow_forward
- Predict and draw the product of the following organic reaction:arrow_forwardNonearrow_forwardRedraw the molecule below as a skeletal ("line") structure. Be sure to use wedge and dash bonds if necessary to accurately represent the direction of the bonds to ring substituents. Cl. Br Click and drag to start drawing a structure. : ☐ ☑ Parrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY